By Ella Chan
INTRODUCTION
The aim of this project was to investigate eugenol (EU) as a bactericidal compound (compound capable of killing bacteria) in saliva (using oral bacteria and probiotics) for gum disease treatment while comparing these to the effects that lactoferrin (LF) and salivary salts had on EU’s ability to kill bacteria. Additionally, other forms of EU were tested, methyl eugenol (ME) and acetyl eugenol (AC-EU) to determine which functional group on the benzene ring is responsible for the bactericidal effect.
While gum disease may seem trivial, it is a condition that is a worldwide burden. Dental caries (tooth decay or cavities) alone has been shown to affect almost all adults at least once in their adult lifetime, and between 60 and 90% of schoolchildren. The distribution of gum disease is localized in the Americas, where diets high in sugar are prevalent, as shown in Figure 1 and Figure 2. However, while gum disease rates in Africa are currently low, the World Health Organization (WHO) predicts that increased exposure to sugar combined with a lack of dental infrastructure will result in a shift in the distribution of the disease.
Figure 1. Dental caries levels of 35-44-year-olds (WHO).
Figure 2. Dental caries levels of 12-year-olds (WHO).
The lack of affordable and effective treatment for gum disease in African regions will result in an increase in the complications associated with the bacterial gum infection. The oral microbiome is the collection of bacteria and organisms in one’s mouth. It home to the “most varied and vast flora” in the body, and is the entrance of two main organ systems in the body: the respiratory and digestive systems. While oral bacterial infections may appear insignificant, they can easily evolve into bacterial endocarditis (BE), a bacterial infection of the heart valves or blood vessels. BE is a life-threatening condition with a mortality rate between 10 and 80%. 8 to 10% of cases are associated oral infections without dental manipulation. Activities such as teeth brushing, chewing gum and eating are common acts of dental manipulation which provoke the movement of oral bacteria and significantly increase the risk of oral infection. When lesions are present in the gums, bacteria have an easy entrance into the bloodstream.
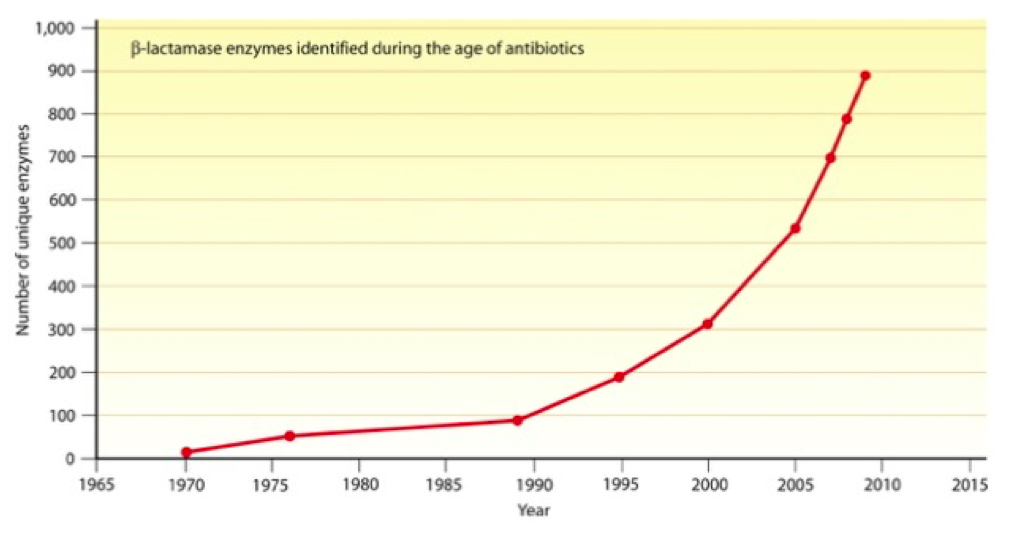
Current preventative treatments of BE include antibiotic prophylaxis (the use of antibiotic therapy) prior to dental procedures with high dental manipulation, such as root canals and routine hygiene visits. However, prolonged use of antibiotic treatments creates an ideal situation for the creation of antibiotic-resistant bacteria. Shown in Figure 3, the number of bacterial enzymes identified that destroy antibiotics, such as β-lactam antibiotics, has exponentially increased since antibiotics were first introduced.
Figure 3. Number of unique β-lactamase enzymes identified since the introduction of the first β-lactam antibiotics. From the American Society for Microbiology.
Alongside oral disease, the WHO also credits antibiotic resistance as “one of the biggest threats to global health, food security and development today”. As antibiotics have been abused in agriculture and medicine, antibiotic resistance has continued to rise. Infections such as pneumonia, tuberculosis and gonorrhea are becoming increasingly irresponsive to antibiotic treatments. Due to the resistance epidemic, alternative bactericidal solutions are vital to treating infections. Gum disease, in particular, is a key candidate for alternative therapies as the timeline for the use of antibiotic prophylaxis is greatly limited due to the rapid increase in antibiotic resistance.
One possible alternative is the use of EU. EU, an oil derived from cloves, has been shown to disrupt the cell membranes of bacteria, such as Salmonella typhi. EU was shown to increase the permeability of the cell membrane, causing the bacteria to rupture. EU has also been specifically used to treat gum disease because it acts to kill the bacteria found in the oral mucous This topic is of current relevance as a 2016 study demonstrated EU’s ability to work against multi-drug resistant Staphylococcus aureus.
The preamble sets the stage for this investigation’s question: Does eugenol act as an effective bactericidal agent in the presence of lactoferrin and other components of saliva?
The bactericidal effect of EU is owed to the behavior of the functional groups on its benzene ring (Figure 4). By understanding which functional group is acting on the bacteria, it would be possible to predict the behavior of other natural products. Specifically, in this study, ME and AC-EU were compared to EU.
Figure 4.
EU could be used to combat bactericidal infections, both orally and dermally, as an alternative treatment to antibiotics. With the growing antibiotic resistance epidemic, it is vital to the health of millions worldwide that new ways to combat bacterial infections are researched. By studying EU and its properties as a bactericidal agent, similar compounds can also be suggested to share effects. Examples of similar compounds include catechol, which is found in maple syrup and has a similar benzene and hydroxyl group structure.
When testing an antibiotic component to be used in a biological system, it is important to study any competing compounds. For saliva, the protein LF is found at high levels when gum disease is present, as saliva uses it as a defence factor against bacterial injuries (such as those from Streptococcus mutans). LF is an iron-binding glycoprotein from the transferrin family that is found in many human secretions (e.g. saliva, milk, tears). It decreases the bacteria’s ability to form biofilms, thus reducing the harm caused to the gums. One study showed that salivary LF had a concentration of 0.008 mg/ml. In addition to LF, saliva also contains various salts (NaCl, CaCl2, KCl and NaHCO3) as well as enzymes (molecules that catalyze reactions such as amylase, lingual lipase, and kallikrein), peptides (proteins), and steroid hormones.
HYPOTHESES
The hypotheses for this study were as follows:
1. EU will produce kill zones with oral and probiotic bacteria (beneficial gut bacteria) due to the OH group on the compound.
2. The EU and LF trials will show larger kill zones than the trials with EU alone because EU and LF have both individually been identified as bactericidal agents.
3. ME and AC-EU will be ineffective at killing bacteria, due to the lack of the OH functional group.
4. The salts will increase the size of the kill zones, due to a synergistic bactericidal action between the salt’s and EU’s respective abilities to kill bacteria.
In order to conduct a scientifically valid test that reflected the research question the following variables were manipulated (Table 1).
Table 1. Table displaying the variables in the experiment.
MATERIALS AND METHODS
EU against Oral Bacteria and Probiotic Bacteria:
Four different concentrations (12.5%, 25%, 50%, 99%) of EU (diluted with mineral oil, as EU is oil soluble) were tested against oral and probiotic bacteria. EU soaked disks of filter paper were created and then placed on inoculated (bacteria exposed) Petri dishes with nutrient agar (Ward’s Science nutrient agar), as shown in Figure 5. The probiotic bacteria used included L. acidophilus, L. casei, and L. rhamnosus. The plates were incubated at 37°C with kill zones measured using a calliper after 32 hours. The different concentrations allowed for exploration of the correct dosage for treatment, while the use of different bacteria types allowed for the tests to cover a broader area of pathogens.
Figure 5. Example Petri dish with EU and oral bacteria.
EU and LF against Oral Bacteria and Probiotic Bacteria:
The above procedure was followed (as for the EU only trial), but in addition to the EU immersed disks, LF (from bovine milk, Sigma-Aldrich) soaked disks were also placed on inoculated Petri dishes in trials with both stacked and adjacent disks. This worked to determine the impact of bodily proteins on the function of EU.
ME and AC-EU against Oral Bacteria and Probiotic Bacteria:
ME, AC-EU and EU were tested against both oral and probiotic bacteria by soaking the disks in 99% solutions of the oil. These disks were placed on Petri dishes and the kill zones were measured as above. This helped determine the jobs of the various functional groups on the treatment.
NaCl, CaCl2, KCl and NaHCO3 against Oral Bacteria and Probiotic Bacteria:
The concentrations of the salts were created to match the highest concentration present in saliva (NaCl: 21 mmol/L, CaCl2: 2.8 mmol/L, KCl: 36 mmol/L, NaHCO3: 25 mmol/L). Disks were soaked in the salt solutions and placed with EU soaked disks. Inoculation, incubation and kill zone measurements were repeated as above. This helped to further determine the impact of bodily minerals on EU’s bactericidal ability.
Safety Protocols and Disposal:
Safety protocols were followed throughout this experiment, including proper eyewear, sterilisation and aseptic technique (the sterile process of inoculating bacteria in a laboratory setting). Proper disposal of Petri dishes was used, including soaking the used agar in a bleach solution and disposing of it as biological waste.
RESULTS AND OBSERVATIONS
Tables 2 and 3 show the results and observations from the experiments. Uncertainties were added to match the calibrations on the devices used.
ANALYSIS OF DATA
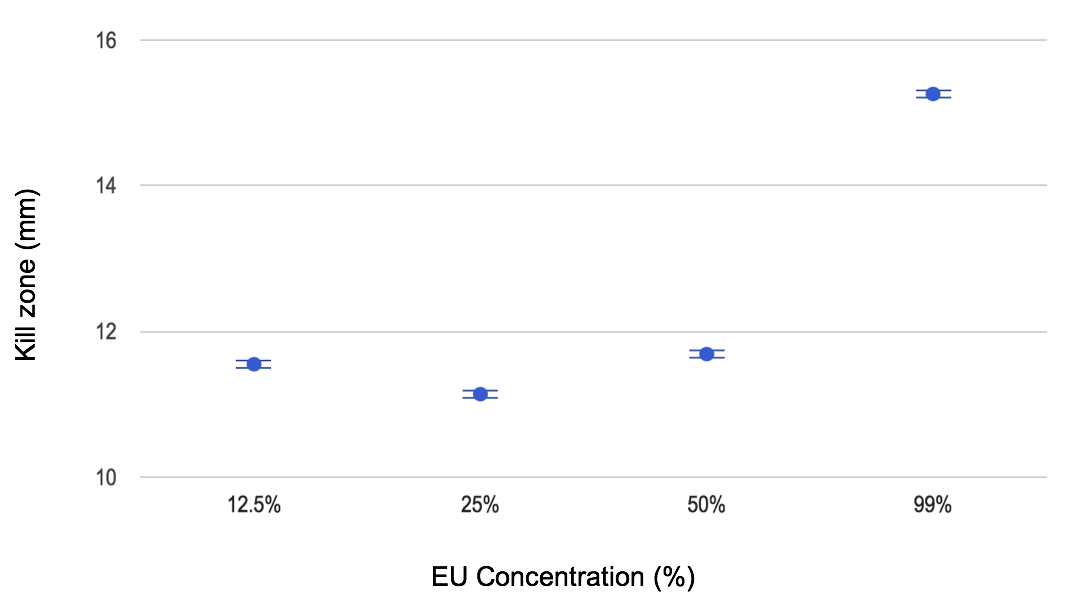
The first trials tested the activity of EU alone against oral bacteria. The kill zones shown ranged from 11.55 ± 0.05 mm to 15.26 ± 0.05 mm on average through all four concentrations (Figure 6). The trials with LF alone showed that there little bactericidal action occurred, with kill zones between 0.00 ± 0.05 mm and 3.75 ± 0.05 mm. The bacteria on these plates also appeared to behave differently, with larger, central colonies of bacteria and uneven distribution of the growth. Similar results were seen in the probiotic trials.
Figure 6. Kill zones measured from the bactericidal effects of varied EU concentrations on oral bacteria.
The trials where both EU and LF were tested on oral bacterium demonstrated varying size colonies on the first three plates; this did not come to any concrete conclusions and would have to be repeated in order to produce significant results. For the trials with EU and probiotic bacteria (Figure 7), there was no growth on the plate. However, when LF was added the kill zone decreased to between 1.70±0.05 mm and 10.60±0.05 mm (depending on the concentration). This provides some evidence to suggest that LF interferes with the bactericidal effects of EU.
Figure 7. Kill zones measured from the bactericidal effects of varied EU concentrations on oral and probiotic bacteria.
The trials where the salts were tested alongside EU showed no oral bacterial growth, except for small colonies near the control disk on the KCl trial. Since the same population of bacteria was used in all trials the lack of growth on these plates could be attributed to a strong bactericidal effect when both are combined. This was similar to the probiotic trials, with the exception of the trial with EU and NaHCO3, which demonstrated an increase in bacterial growth from the plate with EU alone.
The oral bacteria plates with AC-EU and ME showed large growth with no measurable kill zones. The bacteria appeared to encroach on the disks themselves and does not seem to respond to the forms of EU present. The probiotic bacteria plates showed similar results, with far more growth than the plate with the EU.
EVALUATION
Overall, the majority of the results gathered from this experiment were consistent and relevant to the research question. However, there were data points and sections of the experiment that displayed degrees of error.
For the EU only trials the results gathered were consistent and reliable as answers to the research question. Each data point corresponded to the overall trend of an increase in kill zone based on the concentration. However, the kill zone of the 12.5% EU was higher than the 25% EU, leading to the speculation that there was potential for contamination in that concentration of solution. This could be attributed to the cross-contamination of the tools as the disks were transferred from the bottles to the dish, or due to a phase separation between the mineral oil used to dilute the sample and the EU. In order to minimize error like this in the future the concentrations of EU could be remixed before each trial in order to ensure that the concentrations were accurate to the solutions.
For the EU and LF trials, the solutions were tested against both oral and probiotic bacteria. In the oral bacteria trials, the results gathered were mixed and did not come to a conclusive trend. There are several possible reasons for this discrepancy. Firstly, the sample of oral bacteria used was unknown, meaning that the bacteria’s reaction to the LF could have been dramatic based on the type of bacteria present. For example, the bacterial present in the oral sample could have been more susceptible to the antibacterial nature of EU, while probiotics such as Lactobacillus acidophilus may be more resistant to EU. Another possible theory is that when the LF changed biofilms of the bacteria, certain species were affected more than others. This could have led to a change in the way that EU was able to interact with them based on the type of bacteria formed. The variation in colony shape created error in the measurement of the kill zones, as the initial procedure assumed all colonies would be circular. In order to minimize this error, other forms of measurement could have been used. Examples include conducting a serial dilution, a step based dilution of the bacteria allowing for the concentration to be known, and calculating the number of bacteria in a sample or using an area-based technique to determine the space covered by the bacteria. This would help remove the random error associated with the colonies of bacteria present. The probiotic bacteria helped solve both these issues. The kill zones measured reflected the increased concentrations as shown in Figure 7, as the trend was clearly shown. Like the EU only trials, the 12.5% solution did show a different trend, furthering the assumption that the concentration was not accurate.
The salt and other forms of EU trials were as expected as little data discrepancy took place. However, the NaHCO3 showed bacterial growth where the other salts did not. This could be due to the change in the pH of the agar that resulted in the bacteria growing differently, and thus affecting the way EU was able to work. Alternatively, the change in pH could have deactivated EU’s hydroxyl group, decreasing its bactericidal effect.
There are a number of alterations that could take place in order to improve the experiment. These include using saliva-based agar as the material for the Petri dish. This would help more accurately represent how bacteria would grow in an oral environment. Similarly, oral bacteria samples could be taken from many individuals and then combined in order to create a more diverse ecosystem of bacteria for testing.
CONCLUSIONS
When tested against oral bacteria and probiotics EU demonstrated large kill zones, and limited bacterial growth, confirming its behaviour as a bactericidal agent. Further testing showed that the effects of EU appear to be inhibited by the presence of LF on probiotic and oral bacteria. The plates where LF and EU were combined demonstrated large amounts of growth of both the probiotic and oral bacteria with significantly smaller kill zones. These results indicate that if EU were used to help kill bacteria in gum disease, higher concentrations of EU would be required to counteract the high concentrations of LF present.
The plates where salt interacted with the EU showed no bacterial growth when using the oral bacterium, indicating that the EU and the salts have a compounding bactericidal effect. This is possibly a result of the salt causing dehydration of the bacteria. The other forms of EU, AC-EU and ME showed little to no bactericidal effect, indicating that the hydroxyl group (alcohol) in EU is responsible for the beh¬aviour observed. This demonstrates that it is the alcohol group in EU that provides the bactericidal properties and that the other functional groups have little effect.
Based on the results gathered the next steps for this experiment involve testing more components of saliva (such as amylase, lingual lipase, etc.) alongside EU to see if any other compounds limit the bactericidal action. Additionally, other compounds that are based on a phenol structure (a benzene ring with a hydroxyl group) could be tested for similar effects (i.e. Catechol ) to see if other compounds are also viable. The practical applications of this research involve creating an EU lozenge or cream that is applied to the gums of a patient. This would prove to be a solution for gum disease that does not rely on antibiotics. However, while this is promising, the toxicity levels of EU need to be adequately studied before testing and implementation. A proposed concentration is less than 1% EU, which would need to be tested on bacteria in vitro (in a Petri dish) first.
The results of this study could provide the background for formulating a lozenge or chewable gum that treats gum disease and is safe for human consumption. While safe concentrations of EU have not been tested on humans, it has been shown that rats fed EU at 79.3 mg/kg had no adverse effects. However, it is important to note that while previously used in dentistry there have been cases of adverse hypersensitivity to the oil, such as allergic contact dermatitis.
ACKNOWLEDGEMENTS
I would like to especially thank Erin Dallin for mentoring me throughout this project and providing unyielding support. I am also very thankful to Annie Vallance for additional mentorship during this project. I would like to thank Dr. Gabrielle Hager for providing me with the equipment and tools for this project. Finally, I would like to thank Richard Zuk for supporting me through the processes of cultivating and analysing bacterial growth.
REFERENCES
Berluti, Francesca, BScD, Andrea Pilloni, MD, Miriam Pietropaoli, BScD, Antonella Polimeni, MD, DDS, and Piera Valenti, BScD. “Lactoferrin and Oral Diseases: Current Status and Perspective in Periodontitis.” Annali Di Stomatologia (2011). https://www.ncbi.nlm.nih.gov/pubmed/22545184 (accessed June 19, 2017).
Bingham, Eula and Barbara Cohrssen. “Patty’s Toxicology”. New York: Wiley, 2001. Print.
Boron, Walter F and Emile L. Boulpaep. Medical Physiology a Cellular and Molecular Approach. Elsevier, 2016.
Canadian Dental Association. “Gum Disease FAQs.” Canadian Dental Association. https://www.cda-adc.ca/en/oral_health/faqs/gum_diseases_faqs.asp (accessed March 04, 2017).
Dash, Sandeep Kumar, Debasis Mandal, Balaram Das, Sourav Chattopadhyay, Satyajit Tripathy, Durga Pada Dolai, Sankar Kumar Dey, Somenath Roy, and Sandeep Kumar Dash. “Eugenol Provokes ROS-Mediated Membrane Damage-Associated Antibacterial Activity Against Clinically Isolated Multidrug-Resistant Staphylococcus Aureus Strains.” Infectious Diseases: Research and Treatment (2016): 11.
Davies, Julian, and Dorothy Davies. “Origins and Evolution of Antibiotic Resistance.” American Society for Microbiology (Sept. 2010) http://mmbr.asm.org/content/74/3/417.full. (accessed June 15, 2017).
Devi, K. Pandima, S. Arif Nisha, R. Sakthivel, and S. Karutha Pandian. “Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane.” Journal of Ethnopharmacology 130, no. 1 (July 2010): 107-15. doi:10.1016/j.jep.2010.04.025, https://www.ncbi.nlm.nih.gov/pubmed/20435121.
Didry, Nicole, Luc Dubreuil, and Madeleine Pinkas. “Activity of Thymol, Carvacrol, Cinnamaldehyde and Eugenol on Oral Bacteria.” Pharmaceutica Acta Helvetiae 69, no. 1 (July 1994): 25-28. doi:10.1016/0031-6865(94)90027-2. https://www.ncbi.nlm.nih.gov/pubmed/7938073.
Kocaçalışkan, Ismail, Ismet Talan, and Irfan Terzi. “Antimicrobial Activity of Catechol and Pyrogallol as Allelochemicals.” Zeitschrift Für Naturforschung C 61, no. 9-10 (January, 2006). doi:10.1515/znc-2006-9-1004. https://www.ncbi.nlm.nih.gov/pubmed/17137106 (accessed March 04, 2017).
Najjar ,Talib. “Bacterial Mouth Infections.” Medscape. http://emedicine.medscape.com/article/1081424-overview (accessed June 19, 2017).
National Institutes of Health. “Periodontal Gum Disease” National Institute of Dental and Craniofacial Research. https://www.nidcr.nih.gov/OralHealth/Topics/GumDiseases/PeriodontalGumDisease.htm (accessed March 04, 2017).
Petersen, Poul Erik. “Challenges to Improvement of Oral Health in the 21st Century - the Approach of the WHO Global Oral Health Programme.” International Dental Journal 54, no. S6 (12 2004): 329-43. doi:10.1111/j.1875-595x.2004.tb00009.x. https://www.ncbi.nlm.nih.gov/pubmed/15631094 (accessed March 04, 2017).
Sarrami, N., M. N. Pemberton, M. H. Thornhill, and E. D. Theaker. “Adverse Reactions Associated with the Use of Eugenol in Dentistry.” University Dental Hospital of Manchester. (Sept, 2002). https://www.ncbi.nlm.nih.gov/pubmed/12353045 (accessed March 04, 2017).
Scientific American. “How Do Salt and Sugar Prevent Microbial Spoilage?” Scientific American, a division of Nature America, Inc. (February 2006). https://www.scientificamerican.com/article/how-do-salt-and-sugar-pre/ (accessed March 04, 2017).
World Health Organization. “Antibiotic Resistance.” World Health Organization. http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ (accessed July 18, 2017).
About the Author
Ella Chan is a 17-year-old from Victoria, BC who hopes to enter the field of pharmacological research in the future. Ella’s passion for science was ignited through her younger brother’s diagnosis with Nephrotic Syndrome, a potentially-debilitating kidney disease. For the past four years, Ella has been running an educational science YouTube Channel called Sci-Files. She hopes to grow her channel to reach a larger audience and continue to inspire youth to get involved in STEAM. In 2017 she attended the Vancouver Island Regional Science Fair, and then the Canada Wide Science Fair in Regina - where she was awarded a bronze medal for her project.