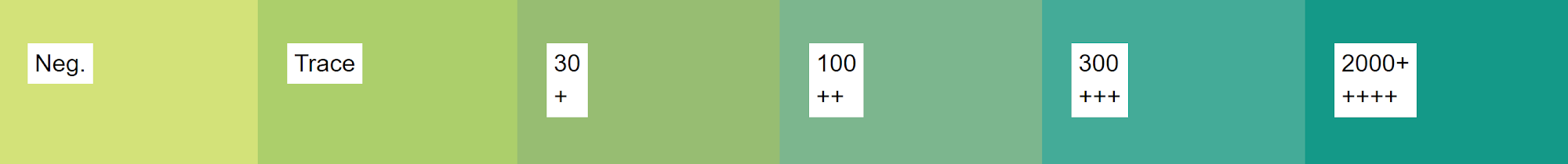Ethan Chan
Age 14 | Victoria, British Columbia
Canada-Wide Science Fair 2019 Excellence Award: Intermediate Silver Medal | Engineering Innovation Award | Ted Rogers Innovation Award | Western University $2,000 Entrance Scholarship
INTRODUCTION
Kidneys filter out unwanted substances and create urine to be excreted, but when the kidneys are damaged or impacted by kidney diseases such as focal segmental glomerulosclerosis (FSGS) or nephrotic syndrome they filter out beneficial substances such as protein into the urine (American Kidney Fund, 2019). For long-term patients with these diseases, daily testing of urine for protein using urinalysis strips is essential (National Kidney Foundation, 2017). Every year in the United States more than 2% of all adults are diagnosed with some form of kidney disease. Furthermore, there are more than 50,000 deaths every year in the United States die from nephritis, nephrotic syndrome, and nephrosis (CDC/National Center for Health Statistics, 2013). Urinalysis strips contain many different square markers that indicate readings ranging from specific gravity to pH that change colour on contact with specific proteins in urine. The protein markers on urinalysis strips are calibrated to albumin, as albumin is the main protein in human urine (Disabled World, 2018).
Due to many outside variables such as colour blindness or uncontrolled ambient light, results are often incorrectly interpreted on urinalysis test strips. These urinalysis results inform clinical decisions such as being hospitalized or having to ingest medicines, such as prednisolone with toxic side effects (National Center for Biotechnology, 2016). Data on many kidney diseases is currently non-existent making digitization of data through electronics invaluable. In the case of nephrotic syndrome, the latest publication of general management protocols were created years ago from insufficient data sets (Bagga, A., 2008), with more recent studies having to rely on historical charts instead of real-time patient data (Sibley, M., et al. 2018).
PURPOSE
Design a cost-effective handheld consumer device to accurately measure the protein found in human urine to accurately measure the protein found in human urine to monitor and/or diagnose kidney diseases. As the protein urinalysis device has to be accurate, it is integral for it to be able to distinguish between defined levels of protein in human urine as outlined in the chart below.
Figure 1. Chart depicting levels of protein in human urine.
These levels being negative, traces, +1, +2, +3, and +4. (Disabled World, 2018). In terms of portability, the device should be able to be easily hand carried, and not be constantly plugged into the wall to function. The device must also be less expensive than the current cheapest protein urinalysis device on the market, found at $190.00 (Coskun, Nagi, Sadeghi, Phillips, & Ozcan, 2013).
PROCEDURES AND MATERIALS
Figure 2. Breadboard prototype.
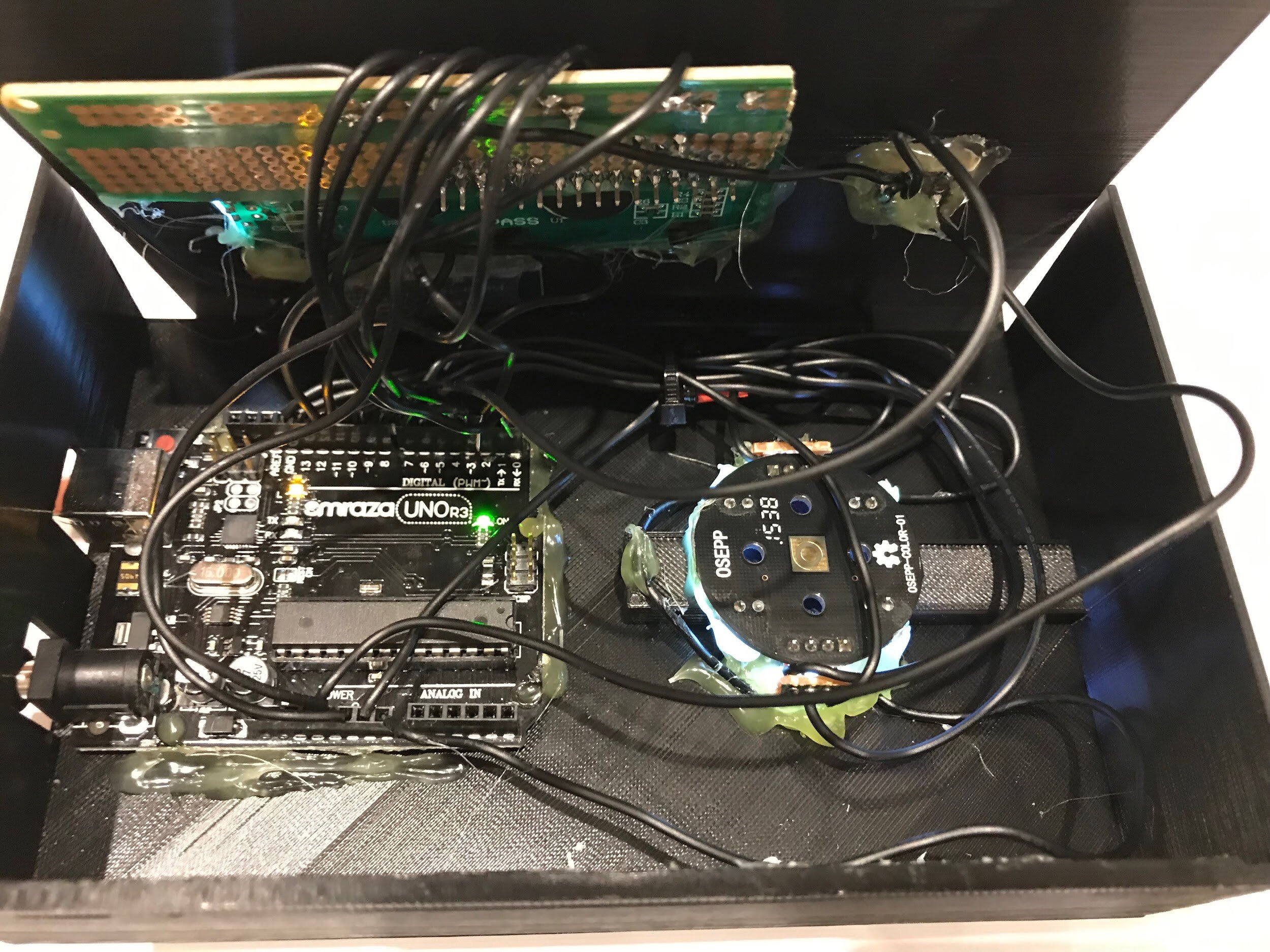
The first step for prototyping the device was to use avrdude, a piece of CLI software developed by Arduino, to flash the correct firmware for the Arduino Uno R3’s ATMega328P chip. A breadboard prototype was then developed by wiring a 16 by 2 LCD screen with pins RS, E, D4, D5, D6, D7 being connected to the respective pins 12, 11, 5, 4, 3, 2 on the Arduino Uno R3. Additionality a colour sensor that functions through blocking various incident lights was added to the device through connecting the pins GND, S0, S1, S2, S3, OUT from the colour sensor to the pins GND, 6, 7, 8, 9, 13 on the Arduino, then a momentary push button with a 5.1k Ohm resistor was also wired in to activate act as a way to activate the sensor (Figure 2).
A basic sketch was then coded in Arduino C that printed values from the colour sensor onto the Serial at a baud rate of 9600. The electronic system was in place while I developed a controlled colour sensing chamber utilising 3D Printing and black filament to get the exact shape. One of the earlier iterations of the controlled colour chamber had a white LED inserted through a bottom to increase the amount of colour hitting the colour sensor chip. This design had a large flaw though, any variation in the placement of the urinalysis strip would result in what the sensor considered a considerable amount of light. A new design consisting of two white LEDs inserted through the sides to increase colour reflection proved to be much more accurate. Another main consideration while prototyping the colour sensing chamber was that various other markers on the urinalysis strip could affect readings from the device. To mitigate this a system that involved inserting rods to block off other indicators was initially put in place. This worked for a while but the system had many flaws in not only being negative from an ease of use standpoint but also couldn’t be implemented due to electronic components in the way. Modeling walls into the two spaces before and after the protein marker on the urinalysis strip turned out to be a much more effective solution as it resulted in the protein marker on the urinalysis strip slightly bending up isolating itself. After the device could differentiate between colour on the protein marker of urinalysis strips a HC-05 Bluetooth Chip that transmits data to and from the Arduino Uno R3 through the Arduino’s onboard default serial port was added to the breadboard (Figure 3).
Figure 3. Urinalysis device.
Along with the HC-05 Bluetooth Chip a mobile application was also developed through the use of MIT App Inventor to transmit data from the sensor to a smartphone, which then displays and caches the data. With the prototype working, it was now time to solder everything together onto stripboards and put it all into a fully 3D printed case for display. With the protein urinalysis device ready to go, a 10.00 mL stock solution was created with a concentration of 250.0 mg of 99% albumin. Different concentrations of albumin were made to approximate the range that would be useful for diagnosis and analysis of kidney disease (0 to 2500 mg/dL). This yielded data points to generate a calibration curve for the sensor. After performing an exploratory data analysis it is visible that there is a logarithmic curve to the red and green colour values in relation to the concentration of albumin in a solution. As the axis would need to be inverted to create a function that can generate a protein value from colour, this means that utilising blue would not be as effective as simply removing it from the calculation. The function that was used works by matching up given colour values into groups between two colour values. From collected data, the machine will know the upper and lower limit of these categories in terms of protein based on colour and apply a linear model for more accuracy in between these points. With the protein urinalysis device now calibrated with the updated C program, albumin solutions were tested again with the same 13 concentrations using a urinalysis strip for each of 10 trials and a disposable micropipette for each concentration to avoid cross-contamination.
RESULTS
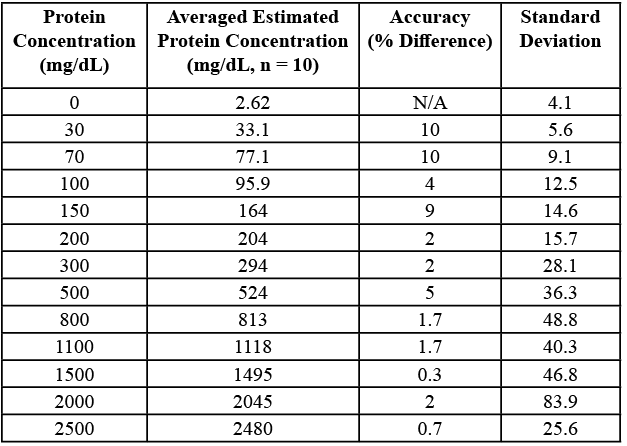
See Table 1 for results.
Table 1. Processed data table for concentration of albumin compared to estimated albumin concentration from device with protein prediction algorithm applied.
DISCUSSION
When the calibrated protein in the urinalysis device was tested with various albumin concentrations, the predicted value was always within 0.5-11.5% of the real protein value of the albumin solution. For example, when testing the 2000 mg/dL sample, the average predicted measurement of protein was 2045.28 mg/dL, which is very close to 2000 mg/dL. From data collected using 99% purity albumin, it is predicted that the device is better than a human at telling the difference between the protein classifications of +2 and +3. This is because there was a notable difference between readings at the levels of 100 mg/dL and 300 mg/dL for the device, and from personal experience, these levels often look identical to the human eye. Differentiation between the levels of +2 and +3 on the defined scale of protein in urine is the most important task of the device due to the fact that at a level of +3 treatment decisions must be made such as administering potentially toxic medication or even hospitalization of the patient. In terms of next steps one of the possible avenues is to create a more miniature portable version of the device. This would be achieved through stripping out various components used to directly interface with the sensor such as the LCD screen and the momentary pushbutton along with minitursing the Arduino Uno R3 into a nano. The end result would be a protein testing device that could fit into your pocket, the only caveat being that it would require a smartphone to activate and measure results. Another direction to take the device would be to make it more accurate by incorporating a spectrometer, while more expensive, would yield an entire graph of data to process into protein data. An important next step is to improve the mobile backend to support aggregating data into a centralised database to collect data to be analyzed for trends. Other features could be implemented into the mobile application including sending reports to a given email, or displaying historical data on various graphs and analyzing it with machine learning algorithms to inform possible treatment options.
CONCLUSION
The protein sensor only needs to be accurate in differentiating between the distinct albumin concentration values of 0, 30, 100, 300, and 2000 mg/dL. From the data collected, the device is easily accurate enough to fulfill the requirements of being accurate enough for medical use. From an economic standpoint, all the components used only cost ~$10, much less than the targeted cost of anything less than $190. At a possible manufacturing level, the cost of the components of the device could be expected to be lower than $10.
ACKNOWLEDGEMENTS
I would like to thank Erin Dallin, Dr. Gabriele Hager, Matt Treble, and Rick Lidstone for their invaluable support and mentorship.
REFERENCES
American Kidney Fund (n.d.). Nephrotic Syndrome. Retrieved from http://www.kidneyfund.org/kidney-disease/other-kidney-conditions/rare-diseases/nephrotic-syndrome/#risk-for-nephrotic-syndrome
Bagga, A. (2008). Revised guidelines for management of steroid-sensitive nephrotic syndrome. Indian Journal of Nephrology, 18(1), 31. doi:10.4103/0971-4065.41289
Coskun, A. F., Nagi, R., Sadeghi, K., Phillips, S., & Ozcan, A. (2013). Albumin testing in urine using a smart-phone. Lab on a Chip, 13(21), 4231. doi:10.1039/c3lc50785h Disabled World. (2018, October 06). Home Urinalysis Test Strip Color Chart and Explanations. Retrieved from https://www.disabled-world.com/calculators-charts/urinalysis.php
National Kidney Foundation. (2017, February 03). Focal Segmental Glomerulosclerosis (FSGS). Retrieved from https://www.kidney.org/atoz/content/focal
Sibley, M., Roshan, A., Alshami, A., Catapang, M., Jöbsis, J. J., Kwok, T., . . . Mammen, C. (2018, 05). Induction prednisone dosing for childhood nephrotic syndrome: How low should we go? Pediatric Nephrology, 33(9), 1539-1545. doi:10.1007/s00467-018-3975-6
BIBLIOGRAPHY
Albumin from chicken egg white A5253. (n.d.). Retrieved from https://www.sigmaaldrich.com/catalog/product/sigma/a5253?lang=en®ion=CA Arduino and HC-05 Bluetooth Module Tutorial. (2019, February 08). Retrieved from https://howtomechatronics.com/tutorials/arduino/arduino-and-hc-05-bluetooth-module-tutorial/
CDC/National Center for Health Statistics (2013, February 18). FastStats - Kidney Disease Retrieved from https://www.cdc.gov/nchs/fastats/kidney-disease.htm
Dejan, Arduino Color Sensing Tutorial - TCS230 TCS3200 Color Sensor. (2018, August 04). Retrieved from https://howtomechatronics.com/tutorials/arduino/arduino-colour-sensing-tutorial-tcs230-tcs3200-colour-sensor/
S. Lee, E. D., Ahn, & U., D. (2013, December 01). Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Retrieved from https://academic.oup.com/ps/article/92/12/3292/1584028
Sandner, L. (2017). BC science connections 8. Provincial Resource Centre for the Visually Impaired. Understanding urine tests. (2016, December 30). Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK279350/
ETHAN CHAN
Ethan Chan is currently a Grade 9 student at Glenlyon Norfolk School in Victoria, BC. He enjoys computer programming, learning various languages such as JS, Java, Python, and C, as well as developing electronic devices with platforms such as Arduino. Diagnosed with nephrotic syndrome at a young age, Ethan has been driven to aid patients with chronic kidney conditions. To achieve this he created a device through the disciplines of coding, 3D printing, and electrical engineering. In his free time, Ethan enjoys network stress testing, rock climbing, and playing video games with friends.