Melody Cheng
Age 17 | Victoria, British Columbia
Canada-Wide Science Fair 2019 Finalist
Introduction
The textile industry consumes a substantial amount of water in its manufacturing processes, used mainly in the dyeing and finishing operation of the plants. The wastewater from the textile plants is classified as the most polluting out of all the industrial sectors, considering the volume generated as well as the effluent composition. In addition, the increased demand for textile products and the proportional increase in their production, and the use of synthetic dyes have together contributed to dye-containing wastewater becoming one of the most substantial sources of severe pollution problems in current times (1).
Azo dyes are the largest group of colourants, constituting 60-70% of all organic dyes produced in the world (2). Some examples of azo dyes include, methylene blue (MB), methyl violet, methyl orange. The success of azo dyes is due to their ease and cost-effectiveness for synthesis compared to natural dyes, and also their great structural diversity, high molar extinction coefficient (3). They have a wide range of applications in the textile, pharmaceutical and cosmetic industries, and are also used in food, paper, leather, and paints. However, some azo dyes can show toxic effects, especially carcinogenic and mutagenic events (4). To avoid these damaging dyes from entering the ecosystem, it is important to develop technologies to remove them from wastewater. One way could be to use silicon dioxide.
Glass wool (silicon dioxide), is a natural product, with great acoustical absorption, high thermal resistance, and environmentally friendly materials. Typically the fiber diameters range from 4 μm to 15 μm and softening point is 675°C (5). In this experiment, I used the absorption property of glass wool and filtered cationic dyes including methylene blue and methyl violet as well as to test the usability of glass wool after the filtration.
EXPERIMENTS
Preparation of Methylene Blue Solutions
Materials: methylene blue power, distilled water, volumetric flask, scale, Spectro-Vis, LoggerPro, pipette, and cuvette.
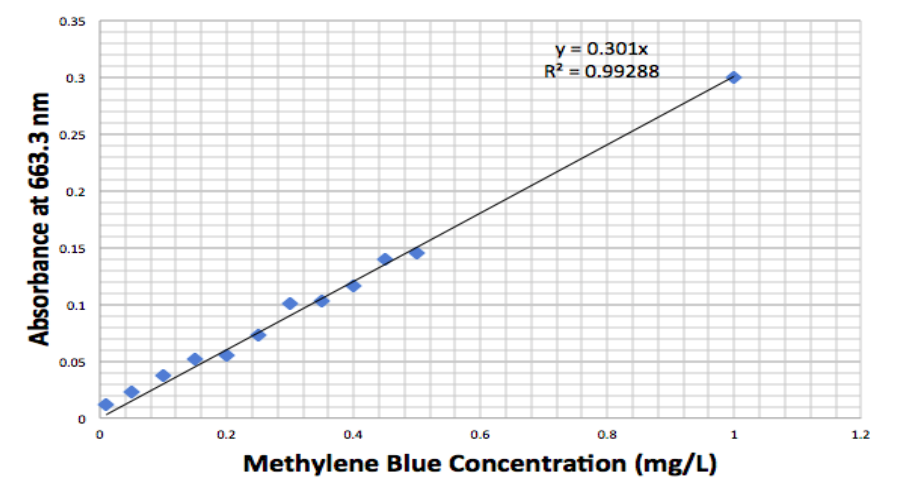
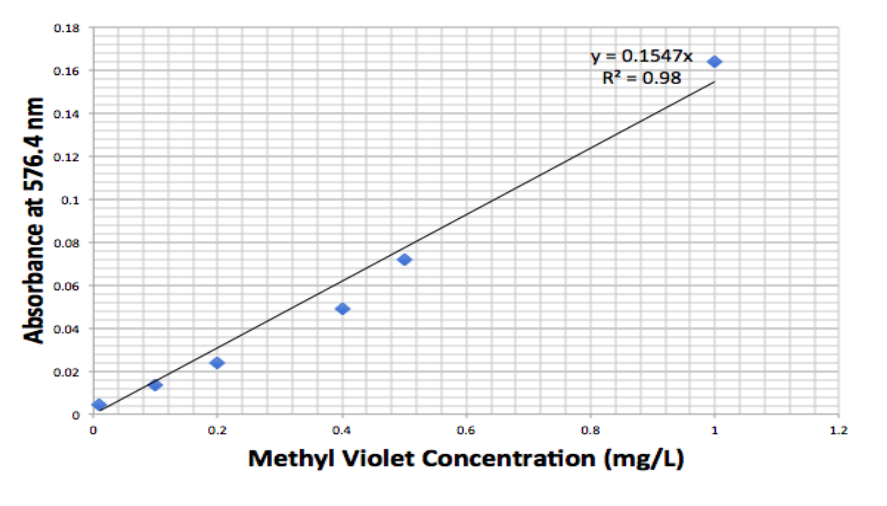
A 3 g/L stock solution of methylene blue was prepared. The solution was diluted to make other concentrations of methylene blue solutions: 0.01, 0.05, 0,1, 0,15, 0.2 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, and 1 mg/L. The absorbance of each concentration of methylene blue was measured at 663.3 nm to make a calibration curve (Figure 1, 2). Similar methods applied to the preparation of methyl violet solutions.
Figure 1. Calibration curve for methylene blue measured with UV-Vis absorption at 663.3 nm.
Figure 2. Calibration curve for methyl violet 2B measured with UV-Vis absorption at 576.4 nm.
Methylene Blue and Methyl Violet 2B Content Analysis after Glass Wool Filtration
Materials: methylene blue and methyl violet 2B solution, pipette, cuvette, Spectro-Vis, LoggerPro, glass pipette, glass wool, and scale.
After examining the results by determining the optimal mass of glass wool in dye filtration, 0.15 g was chosen. 4 mL of 1 mg/L of methylene blue solution was extracted using a pipette to filter through the glass pipette through 0.15 g of glass wool. The filtered solution was then collected in a cuvette to be tested under the Spectro-Vis. This step was repeated for methyl violet 2B. The method was completed in a total of three times for both solutions.
Analysis on Reusability of Glass Wool through Acetic Acid Cleansing
Materials: used glass wool pipette, 8% acetic acid, pipette, cuvette, Spectro-Vis, and LoggerPro
I measured 4 mL of 8% acetic acid using a pipette and poured it through the used glass wool pipette from the methylene blue and methyl violet 2B filtration step. Then the solution was collected to test under the Spectro-Vis at 663.3 nm wavelength
RESULTS AND DISCUSSION
The calibration curves for methylene blue and methyl violet are produced with a high correlation rate and they will be used to convert the absorbance of testing samples to concentration.
To determine whether the dye was simply trapped in the air spaces of the filter, or chemically attached, the absorbance of the methylene blue solution filtered through glass wool and beta-cyclodextrin crosslinked with citric acid (β-CD-CA) was measured. The results show that the absorbance with β-CD-CA is higher than the solution that was only filtered through glass wool without the β-CD-CA. This suggests that methylene blue particles are not just simply trapped inside the air sacs of the glass wool, but there is a chemical bond being formed to enhance the absorptivity of methylene blue on the glass fiber.
In Table 1, on average the glass wool was able to filter 100 % of the methylene blue out of the solution. There is a 90% recovery rate of methylene blue after pouring 4 mL of acetic acid through the glass pipette. Moreover, on average the glass wool was able to filter 85 % of methyl violet out of the solution. There is around 100% recovery rate of the methylene blue in the glass wool after the acetic acid cleansing the pipette.
Table 1. Average quantities of methylene blue and methyl violet measured before and after filtration and after acid washing. This analysis allows for conclusions to be made about the reusability of the glass filters.
I have also tested methyl orange solution by filtering through the glass wool, however, the fiber was only able to filter around 3 % of the chemical. This interaction prompted me for further investigation.
CONCLUSION
Through this novel textile effluent remediation approach, it is found that glass wool fiber is a promising candidate for the removal of methylene blue and methyl violet 2B in water. Moreover, acetic acid successfully removed the absorbed dye particles from the glass fibers making this a reusable and efficient water treatment method. In the future, I will further explore the mechanism of anionic dyes such as methyl orange through changing the pH as well as utilizing beta-cyclodextrin crosslinked with citric acid to enhance the practicality of this remediation technique.
ACKNOWLEDGMENTS
I would like to express my gratitude to my mentors (Erin Dallin and Gabrielle Hager) for guiding me throughout the experiment.
SOURCES CITED
1 Chequer, F. M., Oliveira, G. A., Ferraz, E. R., Carvalho, J., Zanoni, M. V., & Oliveir, D. P. (2013, 01). Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Textile Dyeing and Finishing. doi:10.5772/53659
2 Mondal, S., Purkait, M. K., & De, S. (2018). Advances in Dye Removal Technologies. Springer Singapore.
3 Hettige, A., & Mowjood, M. (2015, 11). Reduction of colour in treated wastewater from textile industry using sawdusts as bio-sorbents. Tropical Agricultural Research,26(4), 666. doi:10.4038/tar.v26i4.8128
4 Bafana, A., Devi, S. S., & Chakrabarti, T. (2011, 12). Azo dyes: Past, present and the future. Environmental Reviews, 19(NA), 350-371. doi:10.1139/a11-018
5 Marhoon, I. (2015). Mechanical and Physical Properties of Glass Wool-Rigid Polyurethane Foam Composites. Al-Nahrain University, College of Engineering Journal, 41-49.
MELODY CHENG
Melody Cheng is currently a grade 12 student at Glenlyon Norfolk School in Victoria, BC. Her curiosity and passion for science and learning have led her to conduct her exploration in labs and participate at the Canada Wide Science Fair three times since grade 9. She founded an organization called STEM Vision that delivers lessons with an emphasis on interactive experiments for underprivileged children in the community centers. During her free time, Melody enjoys connecting with people through volunteering with the city council and organizing MUN conferences. In the future, she hopes to continue to share her love of discovery with others while pursuing a career in biomedical sciences.