TAJRIYAN RAHMAN
Age 17 | St. John’s, NL
Edited by Eric Lind
The term extracellular vesicles (EVs) is generally used to describe a naturally secreted population of heterogeneous membrane-bound spherical shaped organelles found within all mammalian cells (Voichitoiu et al., 2020). These vesicles are composed of a variety of functional molecules suspended in its cytoplasm and lack a functional nucleus. These EVs are released by cells into the extracellular matrix (ECM) to a targeted location. A variety of nucleic acids (DNA, pDNA, mRNA, miRNA and other non-coding RNA), proteins (receptors and enzymes), lipids and many other molecules, which are regulated by its parental cell, are carried within the EV. Through this capacity, EVs serve as excellent transporters of biomolecules to nearby or distant cells within the ECM, creating an efficient method of intracellular communication (Voichitoiu et al., 2020). EVs were once considered to be merely cellular fragments disregarded as wastes in blood clots, but are now understood to be able to participate in intracellular communication of nearby or distant cells (Wolf, 1967). Further advancements in research has allowed EVs to be acknowledged as a crucial component in tumorigenesis, having major roles in metastasis to nearby cells. Thus, EVs modulate the environment both physiologically and pathologically by initiating “niche engineering” which enables cancer cells to utilize the EVs, creating an environment that will be favorable for tumor growth and its sustainability. For opposing cells, such as immune cells, this alteration of the cellular homeostasis creates an unfavorable environment of widespread metastasis. In this review, the many hallmarks of cancers and how they are achieved with EVs to help regulate the spread of a tumor throughout their environment are presented. The key characteristics of EVs which permit proficient means of intracellular communication are also presented.
Keywords: Extracellular vesicles, extracellular matrix, intracellular communication, cellular and molecular biology, cancer hallmarks.
Abbreviations: APCs, Antigen-presenting tumor cells; ApoBDs, Apoptotic bodies; BMMC, Primary bone marrow-derived mast cells; CAFs, Cancer-associated fibroblasts; CTL, Cytotoxic T-lymphocytes; ECM, Extracellular matrix; EGFR, Epidermal growth factor receptor; EVs, Extracellular vesicles; ESCRT, Endosomal sorting complexes required for transport; ERK, extracellular signal-regulated kinase; FasL, Fas Ligand; GDE, glioblastoma-derived exosomes; ILVs, Intramural vesicles; MAPK, Mitogen activated protein kinase; MHC, Major histocompatibility complex; MLCK, Myosin light-chain kinase; MVB, Multivesicular body; MVs, Microvesicles; NK, Natural killer; NPC, Nasopharyngeal carcinoma; PE, Phosphatidylethanolamine; PS, Phosphatidylserine; mRNA, messenger Ribonucleic acid; miRNA, micro Ribonucleic acid; ncRNA, non-coding RNA; PD-L1, Programmed cell death-ligand 1; siRNAs; small interfering RNAs; RNAi, RNA interference; TCR, T-cell receptor; TEX, Tumor-derived exosomes; TME, Tumor microenvironment; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand; VEGFs, Vascular endothelial growth factors.
INTRODUCTION
Cancer is a complex society with interactive populations of both malignant and non-malignant cancer cells. The majority of cancer cells must be able to bypass many set mechanisms from tumor suppressive cells in its microenvironment to be able to form tumors and metastasize. The environment where a tumor begins to develop is termed the tumor microenvironment (TME) and consists of a variety of non-malignant cancerous cells including fibroblasts, extracellular matrix (ECM) signaling molecules, adipose cells, lymphatic and blood cells and immune cells. Cancerous cells must adapt unique ways to breach many set mechanisms of the suppressor cells and be able to invade non-malignant cells. Extracellular vesicles (EVs) have been extensively researched and have been observed to possess key roles in both pathological and physiological condition through procedural modulations such as proliferation, migration, invasiveness, ECM remodeling, recognition, local invasion and breaching of the immune system.
The subgroups of EVs which have been shown to have crucial roles in tumorigenesis include microvesicles (ectosomes, endosomes, and exosomes) and apoptotic bodies. Due to the unreliable methods of tracing EVs by it’s parental cell, the origin of many EVs to remain a mystery. Consequently, scientists have found it difficult to categorize EVs by their specific biogenic pathways. However, scientists have developed a distinctive method to organize EVs by their physical characteristics such as their density, size, biochemical composition, formation and their cell origins. Density is often determined as low, medium or high, and is usually determined by ultracentrifugation (Maas et al., 2017 andThéry, et al., 2018). EVs are often miniature versions of its parent cell, identical in both composition and intracellular activity. Most EVs contain a variety of nucleic acids such as RNA, mRNA, miRNA, ncRNA, DNA, mitochondrial DNA and have intact metabolites, such as amino acids and lipids (Xie et al., 2019). EVs operate within a very sophisticated pathway of communication where they can deliver soluble proteins and a wide variety of coding and non-coding RNAs, which can alter gene expression in the recipient cell. Interestingly, Valadi et al. (2007), found that various functioning mRNAs were found in mouse exosomes (MC/9), human mast (HMC-1) cell lines and primary bone marrow-derived mast cells (BMMC) in mice. Importantly, the mRNA found in the exosomes were capable of dictating protein synthesis in the recipient cell. Moreover Balaj et al. (2011) found single-stranded DNA, including genomic and coding DNA, and transposable elements particularly in exosomes. Most EVs have reflective molecules which are present in their parents’ cells, however many studies have indicated that many of the components may undergo selective processing in which the cell itself chooses what can or can not into the EV (Théry, et al., 2018).
EV Biogenesis
Several studies have demonstrated that cells secrete EVs through the abscission of the plasma membrane through a multitude of pathways discussed below. Exosomes are smaller or equivalent to 100 nm and are called intramural vesicles (ILVs) prior to their release. They are assembled in the multivesicular body (MVB) which resides in the endosomal pathway and is responsible for removal of degraded material from lysosomes. The endosomal network is a membranous compartment that organizes various ILVs into designated pathways of their appropriate destinations (Akers et al., 2013). The MVB gathers such molecules and diverges into two mechanisms: immediate release of ILVs immediately as exosomes via exocytosis or continuing the recycling of the lysosomal components, allowing the cell to recycle molecules within exosomes (Luzio et al.,2010). The formation of exosomes also relies on the fusion of the ILVs with the MVB membrane. During fusion, the endosomal sorting complexes (ESCRT) is required for the formation of ILVs. ESCRT-0 is responsible for enriching the ILV with various proteins crucial for its survival. Other molecules include lipids and sphingomyelins, which are present in the plasma membrane, transmembrane and cytosolic signaling proteins and RNA molecules. ESCRT-I and ESCRT-II are responsible for mediating the release of the ILVs while ESCRT- III is required for the completion of the budding (Hessvik & Llorente, 2017). Once released from the MVB, exosomes may fuse with the plasma membrane of the cell to be released into the ECM (Cocucci & Meldolesi, 2015 and Piper & Katzmann, 2007).
Microvesicles (MVs) are assembled directly at the plasma membrane and range in sizes from 100 nm to 1μm (Cocucci et al., 2009). MVs are produced in clusters at specific regions of the membrane where their biological compositions begin to dictate their content composition of lipids and proteins along with increasing Ca2+ concentration levels. Increasing Ca2+ concentration on the plasma membrane alters the distribution of phosphatidylserine (PS) and phosphatidylethanolamine (PE) present on the inner surface of the membrane where the MVs are produced. Membrane budding is induced by the relocation of PS to the outer membrane. Through the assistance of enzymes, the amplification of Ca2+ ion activates enzymes, such as scramblases and flippases. These enzymes relocate the PS and PE from the inside of the forming vesicles so that they are present on the outer membrane (Zwaal & Schroit, 1997). Ca2+ also contributes to the reorganization of the cytoskeleton via interactions with the membranous actin-myosin network allows vesicles to finally detach from the cell membrane. PS activation promotes the recruitment of the extracellular signal-regulated kinase (ESRK) at the cell membrane which then activates the myosin light-chain kinase (MLCK) necessary for microvesicle release (Muralidharan et al., 2009). The molecular composition of the budding MV is often a replication of what its parent cell was composed of, therefore, a large grouping of MVs may hold plenty of content ((Cocucci & Meldolesi, 2015; Piper & Katzmann, 2007; Cocucci et al., 2009; Zwaal & Schroit, 1997; Muralidharan et al., 2009 and Pap et al., 2009). Once released, MVs remain in the ECM or enter other biological structures and travel throughout the body to distant structures via bodily fluids , which account for the presence of MVs predominantly found in plasma (Camussi et al., 2010). Platelet-derived MVs are termed ‘microparticles’ and MVs derived from leukocytes are termed ectosomes (Martínez et al., 2005). EVs released from platelets upon their adhesion to vessel walls during the processes of blood clotting were initially observed and described by Wolf (1967) and Warren & Vales (1972).
Oncosomes are EVs which are associated with oncogenic molecular cargoes, composed of proteins and nucleic acids. The prefix “onco-” indicates that this vesicle secreted by cancerous cells is of the same phenotype. Oncosome biogenesis is produced in a similar mechanism to how MVs are produced with additional activation pathways which give oncosomes their unique characteristics. The most vital pathways include the epidermal growth factor receptor (EGFR) and the AKT/PKB signaling pathway (Meehan et al., 2016). EGFR is a signaling pathway that is crucial for regulating growth, survival and cell recognition. The AKT/PKB signaling pathway is responsible for allowing foreign DNA to be intercepted into the vesicle by which the oncosomes can promote their survival when they interacts with another cell (Normanno et al., 2006). Cancerous cells release a multitude of EVs, so many groups of similar EVs fall under the term “oncosomes'' however, they vary greatly in their morphology and cargo. A unique group of oncosomes are termed “large oncosomes” and have come to be a seminal discovery for tumorous development, as their biogenesis and release tend to occur in a tumor-specific manner. These large oncosomes are gargantuan in size relevant to their EV counterpart and can range to be greater than 1 to 10 μm. Due to their enlarged size, they can carry much more cargo. Large oncosomes contain similar components of signaling proteins and lipids of tumor-derived exosomes (TEX) and MVs. Although “large oncosomes” are classified under the generic “oncosomes”, they differ substantially and much more evidence needs to be accumulated to provide more specific details (Minciacchi et al., 2015).
Apoptotic bodies (ApoBDS) are some of the largest in the EV family. Their sizes range from 1-5 μm and are secreted as membrane blebs from cells undergoing apoptosis (O'Loghlen, (2017). The role of apoptosis is to destroy cells which are no longer deemed fit for healthy cell function as the cell repairs itself and grows. Apoptosis is an innate mechanism and is present in normal cell function, demonstrating proper immune function. Apoptosis is vital for maintaining homeostasis in a developing cell population within tissues. When cells can no longer function as required, they are self-programed to cessation (Battistelli & Falcieri, 2020 and Elmore, 2007). As the cell prepares to break down, the chromatin in the nucleus begins to condense and much of the components of the cells are separated into vesicles, called apoptotic bodies (ApoBDs). The ApoBDs will begin to make protrusion in the cell membrane, termed a bleb, and detach itself from the cell membrane through the process of budding, entering into the ECM. As the ApoBDs are released, the cell body releases chemical signals via autocrine signaling pathways alerting nearby macrophages of the release of ApoBDs (Atkin-Smith & Poon, 2017). Although the ApopBDs are taken up by macrophages, they can still contribute to intracellular communication by transferring oncogenic molecules into the macrophage, altering the macrophage into a tumor-associated macrophage (TAM). The contribution of TAM is noted to provide necessary survival and growth-inducing aid for the tumor microenvironment; however, the specific mechanisms remain unclear (Kanada et al., 2016).
Irrespective of their origin, all EVs are membrane-enclosed vesicles containing cytosolic components and surface adhesion molecules respective of their cell of origin. Differentiation of each EV class and their specific surface molecules help to influence the recognition and uptake by the cell targeted by the EVs. EVs can be taken in by the target cell endocytic means, such as phagocytosis and pinocytosis or deliver their cargo through fusion with the plasma membrane via detection and internalization by endocytic specific receptor‐ligand interactions (Mulcahy et al., 2014). Once the EV is internalized, its content will be processed and degraded into the cytoplasm and transported into the nucleus or cellular membranes. Interaction between membrane proteins of EVs with receptors of the recipient cell membrane may stimulate intracellular signaling (Demory et al., 2013).
The aforementioned specific and nonspecific mechanisms of EV internalization demonstrate the different pathways of EV‐mediated intercellular communication, through the transferring of enzymes, transcription factors, receptors, oncogene products, and nucleic acids, resulting in the stimulation of the target cells through interaction with cell surface‐expressed receptors (Camussi et al., 2013). These three major subgroups play a key role in the development and establishment of tumors. In this review the general and key mechanisms involved will be further described. To avoid confusion, the term “EV” will be used when describing the entirety of the EV population and for instances where specific EVs are involved along with their designated names.
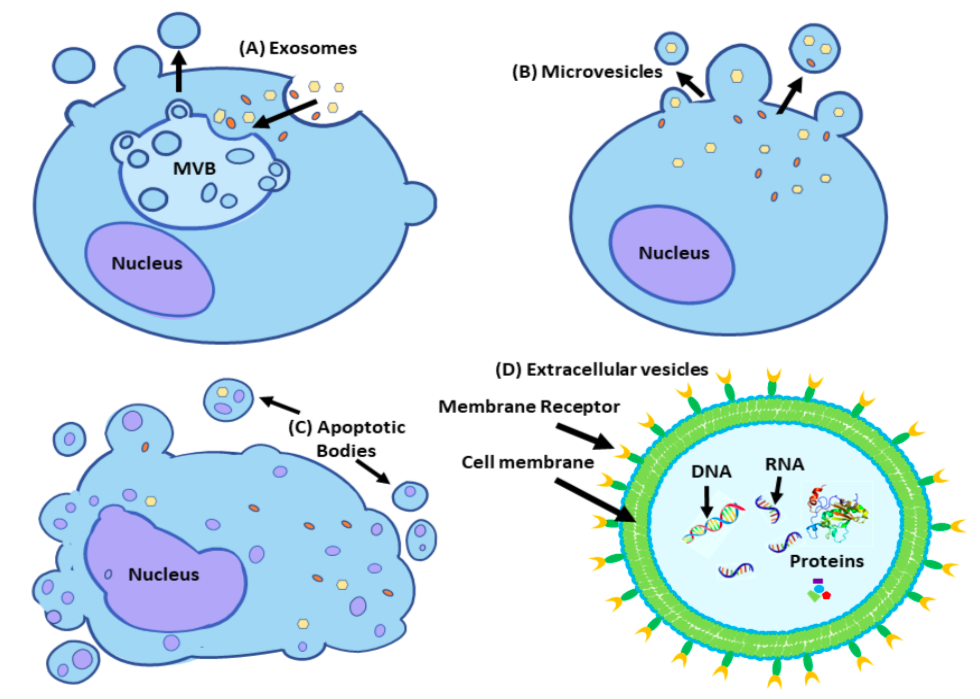
Figure 1: Schematic representation of the main classes of extracellular vesicles (EVs): (A) Exosomes are formed by extracellular molecules taken into the cell through endocytosis and processed to be recycled within the cell's endosomal system. Early endosomes mature into multivesicular bodies (MVBs) where MVBs release intraluminal vesicles (ILVs) into the cytoplasm. Once the ILVs are secreted into the cytoplasm it is called exosomes. (B) Microvesicles (MVs) are secreted at the cell membrane in clusters cell membrane partaking in processes involving ESCRT protein complexes. The MVs are produced as a result of phospholipid redistribution within the plasma membrane often causing clusters of phospholipid molecules in an area on the membrane. The budding process occurs through the contraction of the cytoskeleton that is dependent on actomyosin-dependent mechanisms. (C) Apoptotic bodies (ApoBDs) are released from cells undergoing apoptosis. ApoBDs often contain remnants of the cell’s genomic DNA and are internalized into nearby macrophages. (D) Extracellular vesicles (EVs) is an umbrella term to categorize secreted vesicles from a variety of cells. All EVs are membrane-bound organelles that contain multitudes of genomic DNA (gDNA), mitochondrial DNA, messenger RNA (mRNA), coding and non-coding RNA, and numerous types of proteins and lipids. EVs share the same general function to transport intracellular components to specific cells. Once the components are internalized, the EV can affect the recipient both physiologically and pathologically depending on it’s delivered molecules.
CANCER CELLS AND EV
Tumors develop in tissue environments which are suitable for growth and where the microenvironment is generally undisrupted. The tumor microenvironment (TME) consists of a variety of cell types which interact with one another through different signaling mechanisms. A TME includes cells such as fibroblasts, endothelial and immune cells. As a tumor develops, the cell's intercellular communication becomes distorted, as the proliferation of tumor-derived EVs affect all regions of the niche. For a tumor to effectively develop, it must be able to alter its microenvironment into a pre-metastatic niche; it must attain the several hallmarks of cancer. Cancer hallmarks are distinct actions that are divided into tasks which a TME must achieve to successfully obtain a suitable pre-metastatic niche and further allow cancer dissemination. Such hallmarks include achieving the maintenance of proliferative signaling, evasion of growth suppressors and immune surveillance, resisting cell death, inducing angiogenesis, cell migration and reprogramming glucose metabolic activity in cells (Hanahan & Weinberg, 2011). For tumors to develop, cancer cells must adapt to build mechanisms to benefiting the tumor development. EVs are secreted and used as mechanisms by cancer cells to alter the principle microenvironments into a favorable carcinogenic environment to allow tumors to spread. If utilized effectively, EVs can help the tumor bypass the immune surveillance system and infect other cells with the mutated genome. EVs can also help the tumor achieve its hallmarks needed to help develop a TME through intercellular communications, modulated by various humoral factors.
The most important factor of tumor progression begins with a cancer cells use EVs to mediate cell-to-cell communication. Cancer cells are often organ-specific; thus, the secreted EV are unique to its parental cell (Nicolson, 1982). In a TME, a multitude of different EVs and their individual functions must work together. Upon release from the parental cancer cell, EVs can initiate interaction between cells through local or systematic communication. The EVs are structurally identical to the parental cell, containing the same ligand, receptors, proteins and RNA content. The mutated cargo within the EV is transported to another cell, where the targeted cell takes in the EV through a multitude of uptake mechanisms. The targeted cell and the EV’s ligand-receptor interactions are likely to account for EV recognition and uptake. For EVs transporting RNA and cytosolic content, they must fuse with the recipient cell to release their contents inside the cell’s cytoplasm. Some of the mechanisms of EV uptake include direct EV fusion with the plasma membrane where the EV’s cytosolic content is directly released into the cytoplasm, or the EV is engulfed through endocytosis and the subsequently fuses with the endosomal membrane, releasing its cytosolic content (O'Loghlen, 2017). Delivery of EV cargo can possess responsibility for any physiological activity within the recipient cell. Understanding the difference of how a tumor-derived EV functions versus how a normal cell-derived EV function is crucial to understanding how a tumor may progress. For example, a study was conducted by Morello et al. (2013) profiled miRNA of a cancerous cell and non-cancerous cell where both the secreted EV and its miRNA content were compared. The results demonstrated that the tumor-derived cell had a greater production rate of large oncosome shedding along with slight alterations of its miRNA content between the parent cell and its derived EV. To further compare the miRNA content in the secreted EV, fifty-five shared types of miRNA were identified and the top five expressive miRNAs in both the cancerous cell-derived EV and the non-cancerous cell-derived EV were characterized to have both oncogenic and tumor-suppressive functions. One of the expressed miRNAs includes miR-375, a known biomarker of prostate cancer, was identified in a greater quantity in the cancerous cell-derived EV than its parent cell. Differentiation of miRNA content between the parent cell and the derived EV indicates that the packaging of many miRNA into EVs are either sorted into or excluded through a controlled selective process. The cancerous cell-derived EV that enclosed the miRNAs, specifically EVs that contained miR-1227 consequently affected many biological functions in recipient cells of CAFs such as altering the rate of cell proliferation and epithelial neoplasia (Morello et al., 2013).
Proliferative Signaling and Escape from Growth Suppressors
To achieve metastasis, cancer cells must sustain a high rate of proliferative signaling. Normal cells maintain their cell growth-and-division cycle, maintaining a homeostatic cell population in their cell environment. The cell growth-and-division cycle is maintained trough cell-surface receptors which bind to molecular growth factors present within the cell. As the intercellular receptors complex interact with the molecular growth factors, the mitotic process is resumed through the propagation of receptor-to-growth complex signals. This cycle ensures that any damaged cells which are unfit for a developing environment are removed through apoptosis, and its remnants engulfed by macrophages. However, cancer cells possess well-developed strategies to sustain the cell growth-and-division cycle, greatly expanding their rate of proliferation and ultimately avoiding being forced to apoptosis. One such strategy involves cancer cells initiating autocrine signaling, producing their very own growth factor molecules and recipient ligands (Hanahan & Weinberg, 2011). Another strategy causes cancerous cells to utilize the EV bidirectional transfer of molecules to transport oncogenic molecules to recipient cells, mutating the recipient cell into a cancerous phenotype. Moreover, the original cancerous cell can further utilize bidirectional molecule transfer in EVs by consuming the molecules withing the recipient cell, which further aids in cancerous proliferation. In a study conducted by Ozawa et al. (2018) found that gene expression of EVs derived from HCC1806 cell lines in breast cancer cells was observed upon interaction with MCF10A cells, causing changes to the gene expression of the HCC1806 cells. They observed that a multiple of genes were differentially expressed, demonstrating their involvement in pathways vital for cellular proliferation and survival as the genes interacted with critical pathways. The MAPK (mitogen-activated protein kinases)/ERK (extracellular signal-regulated kinases) pathway is one such pathway and is crucially involved with apoptotic processes. The MAPK/ERK pathway fulfills an important role in integrating paracrine signals from the ECM, such as receiving epidermal growth factor (EGF), which the MAPK/ERK integrates into proliferative cell signaling thereby promoting cell growth and proliferation of the recipient cell.
Evasion of Immune Surveillance
EVs play an important role in maintaining immune cells and their efficiency to work together. Although EVs are crucial for maintaining a healthy population of immune cells, they can also initiate the destruction of immune cells. EVs derived from tumor cells can both suppress and activate the immune system (Maas et al., 2017). While the activation of the immune system will initially reduce tumor growth, cancerous cells generally possess defense mechanisms, evading the immune surveillance.
EVs secreted from cancer cells, immune cells, and other host cells all serve distinct roles in the suppression or expression of tumor proliferation to maintain tumor immunity. Tumor-derived EVs can play a role in tumor progression, metastasis and drug resistance. In a TME, EVs are crucial mediators between the tumor cells and the immune system. Most tumor-derived EVs, particularly exosomes, are enriched with TRAIL (Tumor necrosis factor-related apoptosis inducing ligand), FasL (Fas Ligand), galectin-9 and many more immunoregulatory molecules that can all promote the destruction of T cells and interfere with the natural killer (NK) group 2 member D ligand (NKG2DL)-dependent cytotoxicity of NK cells (Gabrusiewicz et al., 2018).
Cancer cells adapt many mechanisms to interfere with the immune system through EVs to camouflage themselves. Tumor-derived EVs can mediate immunosuppressive activity during tumor progression by suppressing effector immune cells. This is affected by altering the genetic composition of the immune cell via transportation of inhibitory nucleic acids, changing the transcriptome of the cell antigen presentation, and agonist receptor-ligand interactions. This interaction often causes a death-ligand present on the tumor-derived EV to directly influence apoptosis of the immune cell (Lucchetti et al., 2020 and Dörsam et al., 2017).
During immunosurveillance, immune cells (T cells, B cells, macrophages and dendritic cells) are constantly interacting with tumor and stromal cells to prevent tumor growth. Cancerous cells are differentiated from their non-transformed counterparts though their expression of multiple antigens, often the product of aberrant, mutated, or viral protein encoding genes (Schreiber et al., 2011). [38]. Antigen-presenting tumor cells (APCs) secrete EVs to deliver immune-suppressive signaling molecules to regulate the maturation, development, and tumor suppression capacity of targeted immune cells (Xie et al., 2019 and Raposo et al., 1996). A cancer cell with an antigen presentation on its surface can use EVs to stimulate an immunosuppressive niche that protects the tumor from the immune system. For example, nasopharyngeal carcinoma (NPC) cells produce galectin-9, which is a β-galactosidase (lactase), a glycoside hydrolase enzyme. NPC-derived exosomes act as carriers for galectin-9 and are agonists on the Tim-3 receptor. Galectin-9 present on the surface of exosomes is the ligand of the membrane receptor, Tim-3. The interactions between the exosome bearing galectin-9 and the Tim-3 receptor induces apoptosis of the T cell that expresses Tim-3, specifically CD4+T cells and Th1 cells. T cells release γ-interferon to the ECM, causing NPC cells can increase the production and release of galectin-9 in exosomes (Zhu et al., 2005). The exosomes subsequently trigger apoptosis in several T cells. This creates a feedback loop which suppresses the immune system, allowing the NPC to spread (Klibi et al., 2009). Moreover, cancer cells that express major histocompatibility complex (MHC) classes or tumor-associated antigens (TAA) complexes are sought out and destroyed by cytotoxic T cells. To avoid destruction, the T cell must first down-regulate MHC-l expression however, this method is not sufficient enough to escape detection on its own. Through the use of EVs, tumor cells can further escape detection by cytotoxic T-lymphocytes (CTL) and NK cells. The tumor-derived EVs contain a variety of death-ligands which interfere with the cytosolic activity of both the CTL and NK cells cell (Lucchetti et al., 2020). Hedlund et al. (2011) observed that under thermal and oxidative stress, tumor cells increase the secretion of exosomes with NKG2DLs. Exosomes bearing NKG2DLs are soluble and easily bind to their receptor(s) on the NK cell. To escape the detection from NK cells, the tumor cell secretes EVs bearing NKG2DLs to distract the NK cell, enabling the cell attacks the EV rather than the actual tumor cell itself (Hedlund et al., 2011). Furthermore, multiple tumors release EVs with FasL directly to T cells inducing T-cell death as a means of immune escape. FasL, like galectin-9, binds to T cell receptors and induces apoptosis (Czernek & Düchler, 2017). Cancer-derived EVs include several types of immunoregulatory molecules such as FasL, TGF-β, NKG2D ligands, galectin-9 and HSP72 to support the immune escape of cancer cells (Kubiczkova et al., 2012).
In the body, macrophages are utilized as a defense mechanism against pathogens, and are mediators of both the innate and adaptive immune system. However, a tumor-associated macrophage (TAM) can completely change its functional phenotype as it is exposed to the TME and influenced by tumor progressive factors such as hypoxia. The production of TAMs is described as a phenotypic alternation from an M1 to a M2 macrophage, producing protumorigenic functions. Although further research is needed, various studies have suggested that TAMs are different from M2 macrophages both phenotypic and functionally and their origins are preferentially dependent on the notch signaling pathway (Franklin et al., 2014). Currently there is still not enough evidence yet to accurately support this possibility. An experiment conducted by Gabrusiewicz et al. (2018) demonstrated that the internalization of glioblastoma-derived exosomes (GDE) and glioblastoma stem cell line GSC20 exosome in CD14+ monocytes can alter its phenotype into an immune suppressive M2 macrophage phenotype. After the induction of the GDE, the expression of macrophage phenotypes of M1 and M2 and programmed cell death-ligand 1 (PD-L1) were analyzed via flow cytometry. Only the monocytes that had internalized the GDE experienced an enhancement in the upregulation of PD-L1 and many proteins that initiated in transcriptional reprogramming influencing the monocyte’s growth, proliferation rates, and importantly the organization of its actin cytoskeleton. PD-L1 is regulated through the activation of the STAT3 signaling pathway after interaction with IL-10. The upregulation of PD-L1 in the CD14+ monocytes was only present in glioblastoma tissues and was not upregulated in the blood of the glioblastoma patients. STAT3 activation is required for monocytes to initiate diapedesis, a necessary process for tumor metastasis to the circulatory system. The expression of PD-L1 enables the monocyte to bind to programmed cell death protein 1 (PD1) of activated T cells, consequently causing inhibition of the T-cell function in the TME while increasing the survival rate for the monocyte (Matarredona & Pastor, 2019). PD-L1 is a well-known ligand of PD-1 that fulfills a pivotal role in T-cell inhibition and exhaustion. PD-L1 is up-regulated in several cancers to suppress the cytotoxic activity of T-cells and escape the immune systems (Naito et al., 2016).
Tumor-derived EVs normally possess an enriched antigen composition to that of their parental cell. Antigens within tumor-derived EVs presented by the MHC Class I and MHC Class II are used to regulate and enhance immunosuppressive responses in a microenvironment such as proliferation, activation and apoptosis of immunosuppressive regulatory T-cells (Tregs) (Sullivan et al., 2017). Tumor-derived Tregs have a higher suppressive activity that normal Tregs and can secrete exosomes to inhibit DC-activated CD8+T cell and CTL responses of the immune system either directly or indirectly through interactions of APCs with exosomes (Czernek & Düchler, 2017 and Liu et al., 2015). Production of Tregs from mesothelioma-derived EVs expressing membrane TGF-β can inhibit the response of CD8+T cells to interleukin-2 proliferation (Mulcahy et al., 2014 and Clayton et al., 2007). Colorectal cancer-derived EVs can carry TGF-β to nearby T cells changing their phenotype through activation of the Smad signaling pathway. This phenotype changes the function of a tumor-derived Treg. Tumor-derived EVs secrete TGF-β to utilize their ability to directly down-regulate cells of the immune system. Rong et al. (2015) found that breast cancer cells under hypoxic conditions transport TGF- β via EVs to suppress NK cells and T-cells in the TME. Furthermore, TGF-β is found throughout several tumor-derived EVs and is essential for the recruitment of tumor-associated neutrophils (O'Loghlen, 2017 andKubiczkova et al., 2012).
Inducing Angiogenesis
EVs play a crucial role in stimulating the proliferation of endothelial cells, vessel formation and neovascularization in cancer-associated angiogenesis. As tumors progress, the cells expand further away from the vascular system that provides oxygen and nutrients. Tumor cell progression rapidly consumes the available supply of nutrients; thus, a TME relies on an abundance of nutrients for sustainability. Angiogenesis begins as the tumor lacks oxygen supply, resulting in hypoxic development. Consequently, the tumor cells produce signals to ECM, upregulating angiogenic genes such as vascular endothelial growth factors (VEGFs) in response to hypoxia or gene mutations in the tumor tissue. VEGF-A is one of many critical receptor ligands which are expressed by stromal fibroblasts, helping to produce new neovessels supply nutrients to hypoxic regions of the tumor. At the tip of the emerging neovessel are endothelial cells, allowing VEGA-A to guide the neovessel through the environment. The newly formed vessels lead to the proliferation and epithelial-mesenchymal transition (EMT) of the cells, and ultimately tumor metastasis (Pouysségur et al., 2006 and De Luca et al., 2007).
An important question arises regarding VERF content in fibroblasts. Both normal and tumoral stromal fibroblasts contain VEGF; therefore, the mechanism which regulates the expression of VEGF is responsible for dictating whether angiogenesis will occur within a tissue. Angiogenesis is a tumor-specific occurrence, so the factors responsible for angiogenesis may be an interaction between fibroblasts and cancer cells (Ito et al., 2007). One example of a stimulating factor that includes tumor-derived MVs expressing oncogenic EGFR. These MVs are upregulated by endothelial cells that can activate the MAPK and AKT/PKB pathways, consequently triggering an increased expression of VEGF, followed by the autocrine signaling activation of VEGF receptor-2 (VEGF-2). A431 human squamous cell carcinoma cell line-derived MVs contribute to the overexpression of mutant forms of EGFR, such as wide-type EGFR, and human umbilical vein endothelial cells (HUVEC) releases accelerated amounts of VEGF upon exposure to MVs expressing EGFR. Oncogenic EGFR influences the recipient cell to intensify its secretion of VEGF, causing an imbalance in homeostasis and allows the progression of angiogenesis (Al-Nedawi et al., 2009). Additionally, a study by Kucharzewska et al. (2013) observed exosomes from GBM cells under hypoxic conditions induced angiogenesis by modulating the phenotype of endothelial cells and increasing their proliferation. The endothelial cells were programmed by the exosomes to secrete growth factors known to elicit proangiogenic responses, such as EGFR, VEGFR2, and ephrin type-A receptor 2 (EPHA2), that will further stimulate pericytes that consequently activate the AKT/PKB signaling pathways (De Luca et al., 2007 and Al-Nedawi et al., 2009). A study by Grange et al. (2011) discovered that MVs from renal cancer cells expressing the mesenchymal stem cell marker CD105+ retain similar angiogenic properties from its parent cell. CD105+-secreted MVs were found to contain proangiogenic mRNA, miRNA and thereby stimulate angiogenesis. The MVs contained multiple mRNAs for growth factors, such as VEGF, fibroblast growth factor 2 (FGF2), and angiopoietin 1 both of which are all crucial factors for inducing angiogenesis. CD105+ MVs also contained various miRNAs responsible for modulating a variety of functions for cell growth necessary for tumor invasion and metastasis (Grange et al., 2011).
EVs derived from lung cancer cells (A549 or H1299) containing miR-23a and express EGFR enhance the proliferation, migration and promote proangiogenic properties of HUVECs ((De Luca et al., 2007 and Hirata et al., 2002). Such EVs play a key role in radiation resistance and participate in radiation-induced endothelial cell migration. miR-23a transferred into the HUVECs by EVs increases proliferation and migration of the cell by suppressing the tumor suppressor PTEN (Zheng et al., 2017). Furthermore, overexpression of exosomal miR-23a in hypoxic lung cancer targets prolyl hydroxylase and tight-junction protein-12 to enhance angiogenesis (Borzi ey al., 3029).
Metabolic Activity
Metabolism is the combination of anabolic and catabolic activity which results in the production of energy in the form of ATP (adenosine triphosphate), providing energy and metabolites that form in the mitochondria and are crucial for the cells survival (Lu et al., 2015). The main components required for cellular production of ATP oxygen and glucose, both of which are regulated through the process of aerobic respiration. Cancer cells reproduce at greater than normal rates requiring an increase in a higher metabolic demand. However, most cancer cells do not utilize oxidative phosphorylation (OXPHOS) and favor aerobic glycolysis. Although there is plenty of oxygen in many developing TMEs, most cancer cells often do not utilize oxidative phosphorylation (OXPHO), and instead favour aerobic glycolysis. This metabolic activity is termed the “Warburg Effect’ and is considered a hallmark for the majority of cancerous tumor devlopment (Kroemer & Pouyssegur, 2008). Glycolysis is maintained in conditions of fluctuating oxygen tension and ultimately is a drawback for a TME as only two molecules of ATP are produced, instead of thirty-six molecules per cell needed in a normal cell. While normal cells metabolize glucose almost exclusively for energy production, cancer cells boost glucose consumption primarily to provide a constant supply of glycolytic intermediates to satisfy the anabolic need of dividing cells (Lu et al., 2015). As such, developing cancer cells in a TME require a greater amount of glucose uptake to attain energy for survival (Fonseca et al., 2016).
Through the “Warburg Effect”, increased regulation of glycolysis and uptake of glucose are metabolized into pyruvate dehydrogenase (PDH). PDH is converted to lactate and lactic acids which are secreted into the cytoplasm, further influencing the condition of the TME by enhancing tumor invasion (Kroemer & Pouyssegur, 2008). PDH is strongly expressed in tumor-associated stroma and vessels and PDK1, an enzyme that inhibits PDH activity, is hardly prevalent in the stroma, suggesting that the tumor-associated with stroma and vessels depend on aerobic glycolytic metabolism. PDH expression was found to be suppressed in most cancer cells, while PDK1 was strongly expressed in colorectal tumors. This observation demonstrates that the cancer cell has created adaptations in hypoxic niches enabling cell division and subsequent generation to continue to survive in such hypoxic niches (Koukourakis et al., 2006).
Interestingly, interactions between exosomes released from three different breast cancer cell lines (MDA-MB-231, T47DA18, MCF7) and recipient Human Mammary Epithelial Cells activates the production in reactive oxygen species (ROS), inducing autophagy, DNA damage response (DDR), and phosphorylation of p53 in the recipient cell. Oxidative stress from the ROS induces autophagy in recipient stromal cells and produces metabolites to further increase tumorous growth . The altered metabolism from the production of metabolites such as amino acids, lipids, and TCA-cycle intermediates caused the recipient cell to release growth factors that promote tumor metastasis and establishes a bidirectional-cross talk between the cancer cell and its environment (Dutta et al., 2014). Furthermore, EVs secreted by malignant MDA-MB-231 cell lines suppressed glucose uptake by lung fibroblasts and astrocytes through the reprogramming of glucose metabolism in the pre-metastatic niche, increasing the availability of nutrients and glucose for the cancerous cell (Fonseca et al., 2016).
Within the cardiac tissue, cardiomyocytes enhance the production and secretion of exosomes when in conditions of glucose deprivation. The secreted exosomes are targeted to neighboring endothelial cells, another cardiac cell type. Cardiomyocyte-derived cells contain functional glucose transporters and glycolytic enzymes which are internalized by endothelial cells, resulting in an increase of glucose uptake, glycolytic activity and pyruvate production within the cell (Garcia et al., 2015). Additionally, a study by Vallabhaneni et al. (2014) discovered that EVs from human Mesenchymal Stem/Stromal Cells (hMSCSs) contain lactic acid which increased the ability of cancerous cells survive under the hypoxic and nutrient-deprived conditions within a tumor. The secretion of the lactic acid reduced the overall pH of the TME, allowing cancer cells to hide from immune surveillance. Moreover, acidic TME increases the secretion and uptake of EVs between cells in a TME via proton pumps (Atkin-Smith & Poon, 2017).
The majority of EVs contain bioactive lipids and metabolic enzymes, providing them the ability to generate, metabolize and alter their lipid composition, along with its recipient’s composition. Cancerous cell-derived exosomes internalized by non-malignant recipient cells affect their metabolism by evoking elaborate transcriptional modifications in metabolic genes. Tumor-derived exosomes from HD6-4 colon cancer expressing G12V mutant KRAS and decreased expression of integrin beta-1 (ITGB1) were found to inhibit IGTB1 glycolytic and oxidative phosphorylation of exosomes from wild type and KRAS mutant cell lines. Recipient cells that internalized from mutant KRAS cell-derived exosomes showed alterations in their metabolisms (Franklin et al., 2014). Such alterations in the recipient cell metabolism led to an increase in the metabolic flux of the TME which further promotes the proliferation of the cancer cells.
PRE-METASTATIC NICHE
Cancerous tumors develop in niches which support infiltration to the surrounding bodily environment, thereby achieving the hallmarks necessary for tumor dissemination. Initially, the primary tumor cells influence and alter the stromal cells of its microenvironment by promoting physiologically fovourable changes in the expression of extracellular matrix components. The induced environment prior to tumor dissemination is termed the ‘pre-metastatic niche’ and its stromal environment is composed of endothelial cells, ECM components and fibroblasts (Guo et al., 2019 and Kong et al., 2019). Fibroblasts are the most prominent and express metalloproteinase (MMP) and fibronectin (FN) while also producing inflammatory and growth factors (Pankov & Yamada, 2002). Growth factors released by stromal cells promote angiogenesis, allowing tumor-derived factors to be transported through blood flow in the neovessels and increase vascular permeability in the targeted organ (Kong et al., 2019). Moreover, inflammatory factors from stromal cells could attend to the formation of local inflammatory microenvironments (Guo et al., 2019).
Cancer-associated fibroblasts (CAFs) activated by tumors are prevalent in the TME and are one of the most crucial components of the TME which contributes to cancerous progression and metastasis. While the specific molecular mechanisms of CAF in tumor progression is not entirely understood, it is thought that CAFs provide the primary pathway for cancerous cells to communicate with their TME. Many studies have found that CAFs use EVs to communicate with their stromal cell neighbors and aid cancerous cells in perfecting their pre-metastatic niche. The CAF is the most abundant cell type within the TME and originates from either mesenchymal stem cells recruited from bone marrow, resident fibroblasts, or cancerous cells that went through epithelial-mesenchymal transition (EMT). A study conducted by Wu et al. (2020) demonstrated that EVs packaged with LMP1 could convert normal fibroblasts into CAFs through the NF-κB pathways upon internalization. CAFs are characterized by markers such as α of smooth muscle actin α-SMA and additionally express TIMP-1 upregulation, the primary initiator of transforming normal fibroblasts into CAFs. Specifically a study by Zheng et al. 2016 demonstrated that normal liver fibroblasts transformed into CAFs upon the induction of TIMP-1 in HCC through the activation of the IL-6/STAT3 signaling pathway (Zheng et al., 2016).
CAF-derived EVs are great contributors to oral squamous cell carcinoma (OSCC) proliferation, migration, invasion, and survival. The CAF-derived EVs induced invasion in most of the OSCC cell lines but had more intense effects on the HSC-3 cell line including elevated migration, proliferation, and apoptosis rates. Interestingly, CAF-derived EVs modulated the upregulation and downregulation of the gene expression in the HSC-3 cell line. HCS-3 cells that internalized the EVs were shown to arrive at wounds faster than their competing cell lines and to possess a proliferative and invasive EV phenotype, similar to the apoptic phenotype. The CAF-derived EV induced an invasive budding pattern in the OSCC cells during apoptosis and was crucial for remodeling the surrounding ECM with the invasion of the lymphatic and blood vessels while being the initial step for promoting cell dissemination (Dourado et al., 2019). CAF-derived EVs are one of the more capable tumor cells that can effectively conduct remodeling of the ECM. A study on the effect between CAF-derived EVs and salivary adenoid carcinoma (SACC)-derived EVs on lung metastasis found that lung fibroblasts targeted by CAF-derived EVs internalized the EVs via integrin α2β1. This internalization activated the TGF-β signaling pathway, transforming lung fibroblasts through metastatic induction of SACC-derived cells, primarily expressing POSTN. The production of POSTN upon the internalization of CAF-derived EVs is what makes the contribution of CAF-derived EVs to a pre-metastatic niche different from how most tumors or SACC operate and could make POSTN a valuable biomarker specifically for CAF EVs (Kong et al., 2019).
The development of a pre-metastatic niche requires intricate tailoring of the components of the surrounding ECM. BMDCs are crucial for the development of a suitable microenvironment for a primary tumor to metastasize. EVs from cancer cells promote metastatic niche formation by influencing a pro-vasculogenic and pro-metastatic phenotype through elucidating nd mobilizing BMDCs in its TME. Tumor-derived exosomes recruit BMDCs at pre-metastatic sites through the upregulation of proinflammatory molecules. Such exosomes can transfer MET oncogenes from melanoma cells, activating MET signalling proteins, enhancing bone marrow mobilization, and ultimately promoting metastatic progression (Peinado et al. 2012). Furthermore, BMDC-derived EVs carrying miR-92a could activate hepatic stellate cells (HSCs), promote the recruitment of gMDSC in liver cells, and enhance ECM remodeling. This occurs when lung cancer cells alter the gene profile of the BMDCs causing them to secrete EVs with miR-92a to targeted HSCs in the liver miR-92a potentiates HSC activation by TGF-β signaling pathways, promoting lung cancer metastasis (Hsu et al., 2019).
Metastasizing in stromal tissue is key to tailoring a pre-metastatic niche. Tissue organotropism is associated with exosome integrin (ITG) expression profiles. Quantitative mass spectrometry and western blot analysis revealed that ITGα6, ITGβ4, and ITGβ1 are common ITGs present in lung-tropic exosomes. ITGα6 and ITGβ5was present in lung-tropic exosomes. Furthermore, ITGβ3 was present in brain-tropic exosomes. To confirm that ITGs are necessary for exosome tropism, Hoshino et al. 2015 demonstrated how specific stromal cells uptake different exosomes depending on the metastatic organ. For example, cancerous pancreatic exosomes derived from BxPC-3-Lit cells interacted with Kupffer cells, 831-BrT exosomes mainly interacted with CD31+ positive brain endothelial cells, and lung-tropic 4175 exosomes interacted with S100A4-positive fibroblasts. Furthermore, Hoshino et al. (2015) demonstrated that several exosome ITGs can activate the proinflammatory S100 gene. Kupffer cells were exposed to different cell-derived exosomes of a period of two weeks. Analysis of the gene expression by RNA sequencing identified that S100A8 and S100P were upregulated more than the other genes. Several S100 genes in tumor exosome-educated lung WI-38 fibroblasts, including S100A4, S100A6, S100A10, S100A11, S100A13, and S100A16, were upregulated five-fold while the S100 genes in HBEpC treated with 4175-LuT demonstrated no gene expression changes. Lung fibroblasts treated with exosomes proliferated and migrated more and in WI-38 fibroblasts treated with 4175-LuT exosomes showed ITGβ4 signaling proteins. Phosphorylated Src (pSrc) levels were increased in the ITGβ4-dependent manner and its activation could be the main pathway in which tumor-derived exosomes upregulate the expression of proinflammatory S100. Thus, exosome ITGs can influence the upregulation of the expression of pro-inflammatory genes to facilitate tumor metastasis in the distant tissue microenvironment (Hoshino et al., 2015).
CONCLUDING REMARKS
In this review, the recent research and contributing factors towards tumor developments and relation to EVs were summarized. Although a plethora of relevant research regarding EVs and tumorigenesis has been completed in the past decade, the relation between EVs and tumorigenesis still remains a topic of great interest. EVs are beginning to be understood in a greater depth, especially with their partnership among cancers. The discovery of contributing factors to cancer hallmark development through various oncogenes, immune regulator molecules, mutated suppressor genes, angiogenic factors, hypoxia-related molecules, abundant proteins, metabolites and nucleic acids are still being experimented with and philosophized into medicine. In cancer, EVs ensure the development of multiple processes, notably tumorigenesis, pre-metastatic niche formation, metastasis, and drug resistance via bidirectional cross talk between tumoral cells and their surrounding cells in the niche. Recent discoveries have demonstrated that EVs can serve as cargo not only for intracellular transport between cells of biological components, but also artificial cargo inputted for therapeutic purposes. EVs can be harnessed to deliver drugs to target cells and deregulate development in diseases such as cancer, as chemotherapeutic drugs can be delivered directly to tumor sites, increasing the efficiency by which developing tumor niches are eliminated (Jain & Stylianopoulos, 2010 and Adair et al., 2010). A study reported by Tang et al. (2012) demonstrated that EVs derived from apoptotic tumor cells can be isolated and packaged with a selection of chemotherapeutic drugs (Tang et al., 2012). The cell acts as a carrier to deliver the drugs to the tumor cells of the host leading to the elimination of the tumorous cells without any drug-associated side effects. The presence of EVs is found in a plethora of fluids and tissues throughout the body, enables EVs to be used as potential biomarkers due to their unique characteristics and presented markers which can be used as therapeutic targets. The abundant population of EVs could possibly lead to the isolation of EV groups in partnership with the development of tumors for inhibition. Although this process possesses great potential in relation to cancer treatments, this approach has not seen any success due to many unknown factors. The potential surrounding EVs has captivated a vast audience of curious minds, evident by the ever-increasing research surrounding the topic, many questions still remain. By understanding EVs contribution toward facilitating tumorigenesis, metastasis, drug resistance, and by harnessing the EVs capability to transfer their contents, provides the possibility to convert these vesicles into vehicles for the delivery of therapeutic proteins, RNA molecules and drugs.
ACKNOWLEDGEMENTS
The author thanks Ms. Lisa Gallivan, a previous Biology teacher, at Gonzaga High School for providing the inspiration to write this review paper upon completing a science project. The author also thanks to Proton Rahman, Professor of Medicine (Rheumatology), Memorial University of Newfoundland, providing encouragement for this review paper. Furthermore, lot’s of thanks to Dr. Shofiur Rahman, the authors father, and his colleague Professor Paris E. Georghiou, Department of Chemistry, Memorial University of Newfoundland for the encouragement and editing of this review paper. Lastly, the author expresses lots of gratitude for Eric Lind, an undergraduate student at Trent University who has taking his time to provide excellent editing suggestions and for being very supportive of the writing process.
Conflicts of interest: The author declares no conflict of interest.
REFERENCES
Adair, J. H., Parette, M. P., Altınoğlu, E. İ., & Kester, M. (2010). Nanoparticulate Alternatives for Drug Delivery. ACS Nano, 4(9), 4967–4970. https://doi.org/10.1021/nn102324e
Akers, J. C., Gonda, D., Kim, R., Carter, B. S., & Chen, C. C. (2013). Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of Neuro-Oncology, 113(1), 1–11. https://doi.org/10.1007/s11060-013-1084-8
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C., & Rak, J. (2009). Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proceedings of the National Academy of Sciences, 106(10), 3794–3799. https://doi.org/10.1073/pnas.0804543106
Atkin-Smith, G. K., & Poon, I. K. H. (2017). Disassembly of the Dying: Mechanisms and Functions. Trends in Cell Biology, 27(2), 151–162. https://doi.org/10.1016/j.tcb.2016.08.011
Balaj, L., Lessard, R., Dai, L., Cho, Y.-J., Pomeroy, S. L., Breakefield, X. O., & Skog, J. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature Communications, 2(1). https://doi.org/10.1038/ncomms1180
Battistelli, M., & Falcieri, E. (2020). Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology, 9(1), 21. https://doi.org/10.3390/biology9010021
Borzi, C., Calzolari, L., Ferretti, A. M., Caleca, L., Pastorino, U., Sozzi, G., & Fortunato, O. (2019). c-Myc shuttled by tumour-derived extracellular vesicles promotes lung bronchial cell proliferation through miR-19b and miR-92a. Cell Death & Disease, 10(10). https://doi.org/10.1038/s41419-019-2003-5
Camussi, G., C. Deregibus, M., & Tetta, C. (2013). Tumor-Derived Microvesicles and the Cancer Microenvironment. Current Molecular Medicine, 13(1), 58–67. https://doi.org/10.2174/156652413804486304
Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V., & Biancone, L. (2010). Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney International, 78(9), 838–848. https://doi.org/10.1038/ki.2010.278
Clayton, A., Mitchell, J. P., Court, J., Mason, M. D., & Tabi, Z. (2007). Human Tumor-Derived Exosomes Selectively Impair Lymphocyte Responses to Interleukin-2. Cancer Research, 67(15), 7458–7466. https://doi.org/10.1158/0008-5472.CAN-06-3456
Cocucci, E., & Meldolesi, J. (2015). Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends in Cell Biology, 25(6), 364–372. https://doi.org/10.1016/j.tcb.2015
Cocucci, E., Racchetti, G., & Meldolesi, J. (2009). Shedding microvesicles: artefacts no more. Trends in Cell Biology, 19(2), 43–51. https://doi.org/10.1016/j.tcb.2008.11.003
Czernek, L., & Düchler, M. (2017). Functions of Cancer-Derived Extracellular Vesicles in Immunosuppression. Archivum Immunologiae Et Therapiae Experimentalis, 65(4), 311–323. https://doi.org/10.1007/s00005-016-0453-3
De Luca, A., Carotenuto, A., Rachiglio, A., Gallo, M., Maiello, M. R., Aldinucci, D., Pinto, A., & Normanno, N. (2007). The role of the EGFR signaling in tumor microenvironment. Journal of Cellular Physiology, 214(3), 559–567. https://doi.org/10.1002/jcp.21260
Demory Beckler, M., Higginbotham, J. N., Franklin, J. L., Ham, A.-J., Halvey, P. J., Imasuen, I. E., Whitwell, C., Li, M., Liebler, D. C., & Coffey, R. J. (2013). Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Molecular & Cellular Proteomics, 12(2), 343–355. https://doi.org/10.1074/mcp.M112.022806
Dörsam, B., Reiners, K. S., & von Strandmann, E. P. (2017). Cancer-derived extracellular vesicles: friend and foe of tumour immunosurveillance. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1737), 20160481. https://doi.org/10.1098/rstb.2016.0481
Dourado, M. R., Korvala, J., Åström, P., De Oliveira, C. E., Cervigne, N. K., Mofatto, L. S., Campanella Bastos, D., Pereira Messetti, A. C., Graner, E., Paes Leme, A. F., Coletta, R. D., & Salo, T. (2019). Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. Journal of Extracellular Vesicles, 8(1), 1578525. https://doi.org/10.1080/20013078.2019.1578525
Dutta, S., Warshall, C., Bandyopadhyay, C., Dutta, D., & Chandran, B. (2014). Interactions between Exosomes from Breast Cancer Cells and Primary Mammary Epithelial Cells Leads to Generation of Reactive Oxygen Species Which Induce DNA Damage Response, Stabilization of p53 and Autophagy in Epithelial Cells. PLOS ONE. https://doi.org/10.1371/journal.pone.0097580
Elmore, S. (2007). Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology, 35(4), 495–516. https://doi.org/10.1080/01926230701320337
Fonseca, P., Vardaki, I., Occhionero, A., & Panaretakis, T. (2016). Metabolic and Signaling Functions of Cancer Cell-Derived Extracellular Vesicles. International Review of Cell and Molecular Biology, 175–199. https://doi.org/10.1016/bs.ircmb.2016.04.004
Franklin, R. A., Liao, W., Sarkar, A., Kim, M. V., Bivona, M. R., Liu, K., Pamer, E. G., & Li, M. O. (2014). The cellular and molecular origin of tumor-associated macrophages. Science, 344(6186), 921–925. https://doi.org/10.1126/science.1252510
Gabrusiewicz, K., Li, X., Wei, J., Hashimoto, Y., Marisetty, A. L., Ott, M., Wang, F., Hawke, D., Yu, J., Healy, L. M., Hossain, A., Akers, J. C., Maiti, S. N., Yamashita, S., Shimizu, Y., Dunner, K., Zal, M. A., Burks, J. K., Gumin, J., Heimberger, A. B. (2018). Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. OncoImmunology, 7(4). https://doi.org/10.1080/2162402x.2017.1412909
Garcia, N. A., Moncayo-Arlandi, J., Sepulveda, P., & Diez-Juan, A. (2015). Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovascular Research, 109(3), 397–408. https://doi.org/10.1093/cvr/cvv260
Grange, C., Tapparo, M., Collino, F., Vitillo, L., Damasco, C., Deregibus, M. C., Tetta, C., Bussolati, B., & Camussi, G. (2011). Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Research, 71(15), 5346–5356. https://doi.org/10.1158/0008-5472.CAN-11-0241
Guo, Y., Ji, X., Liu, J., Fan, D., Zhou, Q., Chen, C., Wang, W., Wang, G., Wang, H., Yuan, W., Ji, Z., & Sun, Z. (2019, March 11). Effects of exosomes on pre-metastatic niche formation in tumors. Molecular Cancer. https://doi.org/10.1186/s12943-019-0995-1
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hedlund, M., Nagaeva, O., Kargl, D., Baranov, V., & Mincheva-Nilsson, L. (2011). Thermal- and Oxidative Stress Causes Enhanced Release of NKG2D Ligand-Bearing Immunosuppressive Exosomes in Leukemia/Lymphoma T and B Cells. PLoS ONE, 6(2). https://doi.org/10.1371/journal.pone.0016899
Hessvik, N. P., & Llorente, A. (2017). Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences, 75(2), 193–208. https://doi.org/10.1007/s00018-017-2595-9
Hirata, A., Ogawa, S.-ichiro, Kometani, T., Kuwano, T., Naito, S., Kuwano, M., & Ono, M. (2002, May 1). ZD1839 (Iressa) Induces Antiangiogenic Effects through Inhibition of Epidermal Growth Factor Receptor Tyrosine Kinase. Cancer Research. https://cancerres.aacrjournals.org/content/62/9/2554.
Hoshino, A., Costa-Silva, B., Shen, T.-L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., Molina, H., Kohsaka, S., Di Giannatale, A., Ceder, S., Singh, S., Williams, C., Soplop, N., Uryu, K., Pharmer, L., King, T., Bojmar, L., Davies, A. E., Ararso, Y., … Lyden, D. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335. https://doi.org/10.1038/nature15756
Hsu, Y.-L., Huang, M.-S., Hung, J.-Y., Chang, W.-A., Tsai, Y.-M., Pan, Y.-C., Lin, Y.-S., Tsai, H.-P., & Kuo, P.-L. (2019). Bone-marrow-derived cell-released extracellular vesicle miR-92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene, 39(4), 739–753. https://doi.org/10.1038/s41388-019-1024-y
Ito, T.-K., Ishii, G., Chiba, H., & Ochiai, A. (2007). The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene, 26(51), 7194–7203. https://doi.org/10.1038/sj.onc.1210535
Jain, R. K., & Stylianopoulos, T. (2010). Delivering nanomedicine to solid tumors. Nature Reviews Clinical Oncology, 7(11), 653–664. https://doi.org/10.1038/nrclinonc.2010.139
Kanada, M., Bachmann, M. H., & Contag, C. H. (2016). Signaling by Extracellular Vesicles Advances Cancer Hallmarks. Trends in Cancer, 2(2), 84–94. https://doi.org/10.1016/j.trecan.2015.12.005
Klibi, J., Niki, T., Riedel, A., Pioche-Durieu, C., Souquere, S., Rubinstein, E., Le Moulec, S., Guigay, J., Hirashima, M., Guemira, F., Adhikary, D., Mautner, J., & Busson, P. (2009). Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood, 113(9), 1957–1966. https://doi.org/10.1182/blood-2008-02-142596
Kong, J., Tian, H., Zhang, F., Zhang, Z., Li, J., Liu, X., Li, X., Liu, J., Li, X., Jin, D., Yang, X., Sun, B., Guo, T., Luo, Y., Lu, Y., Lin, B., & Liu, T. (2019, December 3). Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Molecular Cancer. https://doi.org/10.1186/s12943-019-1101-4
Koukourakis, M. I., Giatromanolaki, A., Harris, A. L., & Sivridis, E. (2006). Comparison of Metabolic Pathways between Cancer Cells and Stromal Cells in Colorectal Carcinomas: a Metabolic Survival Role for Tumor-Associated Stroma. Cancer Research, 66(2), 632–637. https://doi.org/10.1158/0008-5472.CAN-05-3260
Kroemer, G., & Pouyssegur, J. (2008). Tumor Cell Metabolism: Cancer's Achilles' Heel. Cancer Cell, 13(6), 472–482. https://doi.org/10.1016/j.ccr.2008.05.005
Kubiczkova, L., Sedlarikova, L., Hajek, R., & Sevcikova, S. (2012). TGF-β – an excellent servant but a bad master. Journal of Translational Medicine, 10(1), 183. https://doi.org/10.1186/1479-5876-10-183
Kucharzewska, P., Christianson, H. C., Welch, J. E., Svensson, K. J., Fredlund, E., Ringner, M., Morgelin, M., Bourseau-Guilmain, E., Bengzon, J., & Belting, M. (2013). Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proceedings of the National Academy of Sciences, 110(18), 7312–7317. https://doi.org/10.1073/pnas.1220998110
Liu, Y., Gu, Y., & Cao, X. (2015). The exosomes in tumor immunity. OncoImmunology, 4(9). https://doi.org/10.1080/2162402X.2015.1027472
Lu, J., Tan, M., & Cai, Q. (2015). The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Letters, 356(2), 156–164. https://doi.org/10.1016/j.canlet.2014.04.001
Lucchetti, D., Ricciardi Tenore, C., Colella, F., & Sgambato, A. (2020). Extracellular Vesicles and Cancer: A Focus on Metabolism, Cytokines, and Immunity. Cancers, 12(1), 171. https://doi.org/10.3390/cancers12010171
Luzio, J. P., Gray, S. R., & Bright, N. A. (2010). Endosome–lysosome fusion. Biochemical Society Transactions, 38(6), 1413–1416. https://doi.org/10.1042/BST0381413
Maas, S. L. N., Breakefield, X. O., & Weaver, A. M. (2017). Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends in Cell Biology, 27(3), 172–188. https://doi.org/10.1016/j.tcb.2016.11.003
Martínez, M. C., Tesse, A., Zobairi, F., & Andriantsitohaina, R. (2005). Shed membrane microparticles from circulating and vascular cells in regulating vascular function. American Journal of Physiology-Heart and Circulatory Physiology, 288(3). https://doi.org/10.1152/ajpheart.00842.2004
Matarredona, E. R., & Pastor, A. M. (2019). Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment. Cells, 9(1), 96. https://doi.org/10.3390/cells9010096
Meehan, B., Rak, J., & Di Vizio, D. (2016). Oncosomes – large and small: what are they, where they came from? Journal of Extracellular Vesicles, 5(1), 33109. https://doi.org/10.3402/jev.v5.33109
Minciacchi, V. R., You, S., Spinelli, C., Morley, S., Zandian, M., Aspuria, P.-J., Cavallini, L., Ciardiello, C., Sobreiro, M. R., Morello, M., Kharmate, G., Jang, S. C., Kim, D.-K., Hosseini-Beheshti, E., Guns, E. T., Gleave, M., Gho, Y. S., Mathivanan, S., Yang, W., … Di Vizio, D. (2015). Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget, 6(13), 11327–11341. https://doi.org/10.18632/oncotarget.3598
Morello, M., Minciacchi, V., de Candia, P., Yang, J., Posadas, E., Kim, H., Griffiths, D., Bhowmick, N., Chung, L., Gandellini, P., Freeman, M., Demichelis, F., & DiVizio, D. (2013). Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle, 12(22), 3526–3536. https://doi.org/10.4161/cc.26539
Mulcahy, L. A., Pink, R. C., & Carter, D. R. (2014). Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles, 3(1), 24641. https://doi.org/10.3402/jev.v3.24641
Muralidharan-Chari, V., Clancy, J., Plou, C., Romao, M., Chavrier, P., Raposo, G., & D'Souza-Schorey, C. (2009). ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Current Biology, 19(22), 1875–1885. https://doi.org/10.1016/j.cub.2009.09.059
Naito, Y., Yoshioka, Y., Yamamoto, Y., & Ochiya, T. (2016). How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cellular and Molecular Life Sciences, 74(4), 697–713. https://doi.org/10.1007/s00018-016-2346-3
Nicolson, G. L. (1982). Cancer metastasis. Biochimica Et Biophysica Acta (BBA) - Reviews on Cancer, 695(2), 113–176. https://doi.org/10.1016/0304-419X(82)90020-8
Normanno, N., De Luca, A., Bianco, C., Strizzi, L., Mancino, M., Maiello, M. R., Carotenuto, A., De Feo, G., Caponigro, F., & Salomon, D. S. (2006). Epidermal growth factor receptor (EGFR) signaling in cancer. Gene, 366(1), 2–16. https://doi.org/10.1016/j.gene.2005.10.018
O'Loghlen, A. (2017). Role for extracellular vesicles in the tumour microenvironment. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1737), 20160488. https://doi.org/10.1098/rstb.2016.0488
Ozawa, P. M., Alkhilaiwi, F., Cavalli, I. J., Malheiros, D., de Souza Fonseca Ribeiro, E. M., & Cavalli, L. R. (2018). Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Research and Treatment, 172(3), 713–723. https://doi.org/10.1007/s10549-018-4925-5
Pankov, R., & Yamada, K. M. (2002, October 15). Fibronectin at a glance. Journal of Cell Science. https://doi.org/10.1242/jcs.00059
Pap, E., Pállinger, É., Pásztói, M., & Falus, A. (2009). Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflammation Research, 58(1), 1–8. https://doi.org/10.1007/s00011-008-8210-7
Peinado, H., Alečković, M., Lavotshkin, S., Matei, I., Costa-Silva, B., Moreno-Bueno, G., Hergueta-Redondo, M., Williams, C., García-Santos, G., Ghajar, C. M., Nitadori-Hoshino, A., Hoffman, C., Badal, K., Garcia, B. A., Callahan, M. K., Yuan, J., Martins, V. R., Skog, J., Kaplan, R. N., … Lyden, D. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine, 18(6), 883–891. https://doi.org/10.1038/nm.2753
Piper, R. C., & Katzmann, D. J. (2007). Biogenesis and Function of Multivesicular Bodies. Annual Review of Cell and Developmental Biology, 23(1), 519–547. https://doi.org/10.1146/annurev.cellbio.23.090506.123319
Pouysségur, J., Dayan, F., & Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441(7092), 437–443. https://doi.org/10.1038/nature04871
Raposo, G., Nijman, H. W., Stoorvogel, W., Liejendekker, R., Harding, C. V., Melief, C. J., & Geuze, H. J. (1996). B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine, 183(3), 1161–1172. https://doi.org/10.1084/jem.183.3.1161
Rong, L., Li, R., Li, S., & Luo, R. (2016). Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncology letters, 11(1), 500-504. https://doi.org/10.3892/ol.2015.3841
Schreiber, R. D., Old, L. J., & Smyth, M. J. (2011). Cancer Immunoediting: Integrating Immunity's Roles in Cancer Suppression and Promotion. Science, 331(6024), 1565–1570. https://doi.org/10.1126/science.1203486
Sullivan, R., Maresh, G., Zhang, X., Salomon, C., Hooper, J., Margolin, D., & Li, L. (2017). The Emerging Roles of Extracellular Vesicles As Communication Vehicles within the Tumor Microenvironment and Beyond. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00194
Tang, K., Zhang, Y., Zhang, H., Xu, P., Liu, J., Ma, J., Lv, M., Li, D., Katirai, F., Shen, G.-X., Zhang, G., Feng, Z.-H., Ye, D., & Huang, B. (2012). Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nature Communications, 3(1). https://doi.org/10.1038/ncomms2282
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin-Smith, G. K., Ayre, D. C., Bach, J.-M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., Zuba-Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. https://doi.org/10.1080/20013078.2018.1535750
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659. https://doi.org/10.1038/ncb1596
Vallabhaneni, K. C., Penfornis, P., Dhule, S., Guillonneau, F., Adams, K. V., Mo, Y. Y., Xu, R., Liu, Y., Watabe, K., Vemuri, M. C., & Pochampally, R. (2014). Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. https://doi.org/10.18632/oncotarget.3211
Voichitoiu, A.-D., Mihaela Radu, B., Pavelescu, L., Cretoiu, D., Teona Deftu, A., Suciu, N., & Maria Cretoiu, S. (2020). Extracellular Vesicles in Cancer. Extracellular Vesicles and Their Importance in Human Health. https://doi.org/10.5772/intechopen.85117
Warren, B. A., & Vales, O. (1972). The release of vesicles from platelets following adhesion to vessel walls in vitro. British journal of experimental pathology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2072544/
Wolf, P. (1967). The Nature and Significance of Platelet Products in Human Plasma. British Journal of Haematology, 13(3), 269–288. https://doi.org/10.1111/j.1365-2141.1967.tb08741.x
Wu, X., Zhou, Z., Xu, S., Liao, C., Chen, X., Li, B., Peng, J., Li, D., & Yang, L. (2020). Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Letters, 478, 93–106. https://doi.org/10.1016/j.canlet.2020.03.004
Xie, F., Zhou, X., Fang, M., Li, H., Su, P., Tu, Y., Zhang, L., & Zhou, F. (2019). Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Advanced Science, 6(24), 1901779. https://doi.org/10.1002/advs.201901779
Zheng, X., Xu, M., Yao, B., Wang, C., Jia, Y., & Liu, Q. (2016). IL-6/STAT3 axis initiated CAFs via up-regulating TIMP-1 which was attenuated by acetylation of STAT3 induced by PCAF in HCC microenvironment. Cellular Signalling, 28(9), 1314–1324. https://doi.org/10.1016/j.cellsig.2016.06.009
Zheng, Y., Liu, L., Chen, C., Ming, P., Huang, Q., Li, C., Cao, D., Xu, X., & Ge, W. (2017). The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ, 5. https://doi.org/10.7717/peerj.3627
Zhu, C., Anderson, A. C., Schubart, A., Xiong, H., Imitola, J., Khoury, S. J., Zheng, X. X., Strom, T. B., & Kuchroo, V. K. (2005). The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunology, 6(12), 1245–1252. https://doi.org/10.1038/ni1271
Zwaal, R. F. A., & Schroit, A. J. (1997). Pathophysiologic Implications of Membrane Phospholipid Asymmetry in Blood Cells. Blood, 89(4), 1121–1132. https://doi.org/10.1182/blood.V89.4.1121
ABOUT THE AUTHOR
Tajriyan Rahman
My name is Tajriyan Rahman and I am currently a grade 12 student attending Gonzaga Regional Highschool in St. John’s NL. I am a very curious and always open-minded about anything and love to learn! As an avid student interested in STEM, I am always looking for opportunities to learn and expand my knowledge. Recently, I have found great interest in the topic of cancer metastasis and our future approaches for cancer research and treatments. Currently, I am a SHAD fellow at McMaster University and hope to start a career where I can further indulge in engaging my curiosity and love for science! Some of my personal hobbies include reading, playing the clarinet, painting and volunteering for my community!