Natalia Papaj
she/her | age 14 | Strathroy, ON
Edited by Kat Kabanova
INTRODUCTION
1.1 Soil Pollution
Pollution in soil is a result of the presence of toxic chemicals (pollutants or contaminants) of high enough concentrations to threaten the health or ecology of humans and other organisms. Even when soil contaminant levels are not high enough to pose a threat, the soil can still be polluted when the levels of contaminants exceed their naturally occurring levels (Everything Connects, 2013).
There are four types of soil pollutants. The first type is biological agents. Biological agents are organisms that are naturally present in the environment but are dangerous. Bacteria, viruses, fungus, and other microbes, together with the toxins they produce, are examples of biological agents. They are capable of having a negative impact on human health in a number of different ways, from very modest allergic reactions to major medical conditions leading to death. Another soil pollutant type is agricultural. This is when the environment is contaminated by naturally occurring or chemical agricultural by-products. The next type of soil pollutant is radioactive. When any process emits radiation into the environment, it is classified as radioactive pollution. The last type of soil pollutant is industrial waste. This refers to physical waste that is not disposed of properly (Plastech Plus Inc, 2022).
1.2 Acid Precipitation
One of the many types of soil pollution is acid precipitation. Acid precipitation is defined as “any form of precipitation that contains high levels of nitric and sulfuric acids” (Environmental Protection Agency). Although commonly known as acid rain, it can exist in the form of snow, fog, rain, hail, and even small bits of dry material that rest on Earth. Acidity and alkalinity are measured on a pH scale where 7.0 is neutral. The lower a matter's pH, the more acidic the matter is. If the matter has a higher pH, it is more alkaline. Normal rain possesses a pH of around 5.6. The reason that normal rain is slightly acidic is that carbon dioxide is dissolved into it, creating weak carbonic acid (United States Environmental Protection Agency, n.d).
Figure 1.1: This image shows the acidity of acid rain against other common household products. (EPA, 2016)
Acid rain results from a different chemical reaction. It starts with particles like sulfur dioxide and nitrogen oxides being released in the environment. Humans release these particles the most in smokestacks and car engines. These chemicals rise high into the atmosphere to mix and react with other particles, such as water and oxygen. This forms the acidic pollutants commonly known as acid rain. Nitrogen oxides and Sulfur dioxide dissolve quickly in water and are, in most cases, carried out to another region of the Earth by the wind (United States Environmental Protection Agency, n.d).
1.3 Effects of Acid Rain
Acid Rain is tremendously damaging to forests. When it soaks into the ground, it can dissolve nutrients, including (but not limited to) magnesium and calcium, which trees and other plants need to survive. Additionally, acid rain can cause aluminum to be released into the soil. Plants have difficulties taking up water from aluminum-polluted soils. The plants that are at most risk of acid rain are those at higher elevations, because they are exposed to denser acidic clouds, fog, and humidity. Acidic clouds and fog contain more acid than acidic rain and snow. The acid strips important nutrients from plant leaves, making it more susceptible to infections, insects, and cold weather damage (United States Environmental Protection Agency, n.d).
Figure 1.3: This graph shows the effect that acid rain has on the average growth of plants with different levels of pH (Takacs Pomegranate, n.d).
Normally, the pH level of unpolluted lakes and streams would be around 6.5. As a result of acid rain, many lakes and streams around the world have much lower pH levels. Additionally, aluminum released into the soil eventually ends up in lakes and streams. Phytoplankton, mayflies, rainbow trout, smallmouth bass, frogs, spotted salamanders, crayfish, and other creatures within the food web can be killed by both the acidity levels and the aluminum concentrations. By making their way through the food chains, these contaminants kill off entire ecosystems, including the plants and the animals that eat them (United States Environmental Protection Agency, n.d).
In addition to affecting the environment, acid rain also affects humans. Respiratory disorders may develop or worsen as a result of airborne sulfur dioxide and nitrogen oxides. Breathing may become difficult for people with respiratory disorders such as asthma and chronic bronchitis. The tiny particles occurring because of acid rain can also cause or worsen these breathing problems. Moreover, nitrogen oxides contribute to the formation of ground-level ozone. Ground level ozone is not emitted directly into the atmosphere but is instead produced through chemical reactions between nitrogen oxides and volatile organic compounds. In the presence of sunlight, pollutants released by automobiles, power plants, industrial boilers, refineries, chemical plants, and other sources chemically react with the ground level ozone. If ground level ozone reaches human lungs, respiratory problems such as pneumonia and bronchitis may develop. As such, acid rain itself does not cause health problems, but the tiny airborne particles and/or the inhaled ozone can pose a serious health risk. A person does not suffer any more harm from swimming or walking in an acidic puddle than walking or swimming in clean water (United States Environmental Protection Agency, n.d).
1.4 Phytoremediation
Researchers are currently investigating a new method of soil and water decontamination called phytoremediation, which takes advantage of certain plants' ability to decontaminate soils and water. Certain plants can decontaminate in a reasonable amount of time at a reasonable cost with minimal disruption to the environment. For soil cleanup, special plants that remove or render harmless contaminants are planted on contaminated sites. The similarity of phytoremediation techniques to agricultural practices makes it particularly well suited for the treatment of large expanses of moderately contaminated soil, on which excavation is not feasible. In ponds or bioreactors, a similar procedure can be used to treat groundwater and wastewater (Negri and Hinchman, 1996).
Figure 1.4: This image shows how a plant absorbs pollutants from different environments. (Research Gate, n.d)
A plant's nutrition and growth depend on water, which makes up 70-90% of the plant's weight. Plants’ roots take water in, their tissues transport it, and their leaves evaporate it through stomata. Photosynthesis and the use of inorganic nutrients such as nitrogen and phosphorus are made possible by water. Scientists developed a waste treatment method that utilizes green plants to reduce the amount of wastewater which is brought to the surface along with gas from natural gas wells. As well as containing dissolved salts, it contains contaminants such as hydrocarbons and heavy metals. The treatment of such water involves reducing its volume and removing contaminants (Negri and Hinchman, 1996).
1.5 Sansevieria trifasciata
Snake plant or mother-in-law tongue is the common name for Sansevieria trifasciata, a species of flowering plant native to tropical west Africa, including Nigeria and the Congo. Because it can adapt to so many conditions, this hardy indoor plant is a favorite with gardeners today. The leaves of snake plants are stout, upright, and sword-like with gray, silver, or gold bands or edges. Modern and contemporary interior designs are made easy with snake plants because of their aesthetic look (Figure 3.1). Snake plants are also resistant to drought, have few insect problems, and can survive low light levels (Rupam, 2019).
I chose snake plants for this experiment because they can survive hot and dry climates. This makes these plants perfect for removing acid rain from a large portion of Africa, a large part of which is acid-sensitive (Fig.1.5).
Figure 1.5: Areas in the world where acid affects the environment (Britannica, n.d).
HYPOTHESIS
No testing of snake plants removing acid pollution has been documented. However, other research has been conducted on different plants. Sebertia acuminata, a tree known from New Caledonia, can accumulate more than 20% of its dry weight in nickel through its latex. Alina Kabata-Pendias reported other interesting accumulations, including greater than 10% zinc accumulation by penny cress (Thlaspi calaminare), 10% nickel accumulation by alyssum (Alyssum bertolonii), and up to 3% chromium concentration in Pimela suteri and broom tea tree (Leptospermum scoparium). Uncinia leptostachya and Coprosma arborea removed up to 3% uranium from the soil, according to Kabata-Pendias. The paper birch (Betula papyrifera) has been reported to remove up to 1% mercury (Negri and Hinchman, 1996).
Several NASA studies have shown that snake plants improve indoor air quality and reduce toxins. As a medicinal plant, snake plants eliminate indoor pollutants (including formaldehyde and benzene) and help filter out unwanted substances (such as xylene, trichloroethylene, toluene, and ammonia) from the air. Snake plants can remove up to 87% of air pollutants at night. These plants can also reduce the amount of nitrate ions in the air (Scotts, 2021).
MATERIALS & METHODS
Three Sansevieria zeylanica (Ceylon Bowstring Hemp) snake plants were planted about 57 centimeters tall in a flower pot about 16 centimeters in diameter and 14 centimeters high. 8.8L of Miracle-Gro Moisture Control Potting Soil was used to fill the pot 11/14 full. SunBlaster Horticultural Light, 48 W 6400 K Full spectrum High Output light was placed 10 centimeters from the top of the plants. Plants A, B, and Control were labeled. The pH of the soil was measured using a Luster Leaf Rapitest pH Meter. To simulate the effects of acid rain, a dropper was used to add 6ml Nitric Acid Solution, ACS Grade, 15.8M, 70% to a granulated cylinder. One liter tap water was also poured into the same granulated cylinder. This solution was verified using pHydrion Papers Oto Dual Range Jumbo to have a pH of about 4. Plants A and B were watered with 100ml of the acid rain solution. Plants A and B were watered with 100ml of the acid rain solution every six days. The control plant was watered with 100ml of tap water, also every six days. The water was distributed evenly around the soil. Soil pH was tested two days after watering, then again the following day from the root using a Luster Leaf Rapitest pH Meter.
Figure 3.1: This shows my setup.
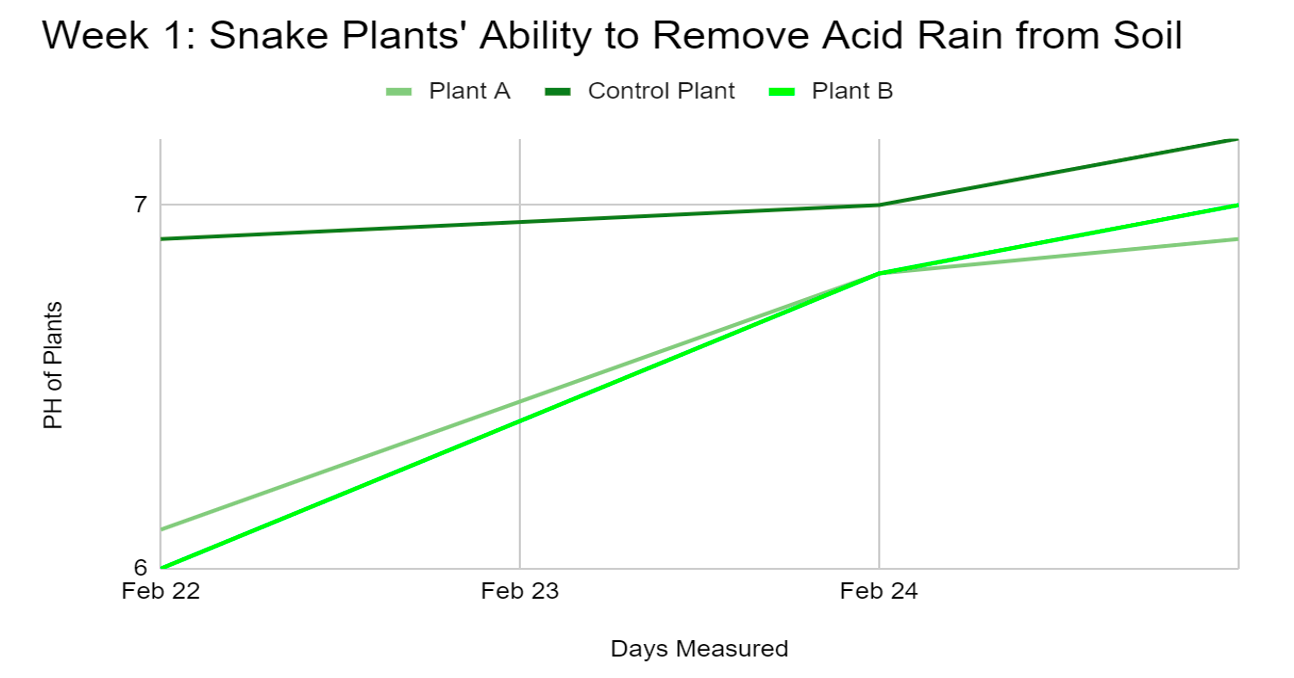
Figure 4.1: The graph shows the first week in which three snake plants remove acid rain from soil.
After only 4 days of testing the snake plants removed almost all the acid rain from the soil. At the beginning of the experiment on February 22, 2022, the pH of the soil was 6.8 for all plants (A, B and Control). When watering plants A and B with acid rain both their pHs dropped. Plant B had a pH of 6, and plant A had a pH of 6.1. Two days later the plants’ soil pH was measured again. The control plant’s pH was neutral (7). Plants A and B were back to their initial pH of 6.8. When measured again the next day (February 25 (?)), the pH of Plants A and B had increased to almost neutral - Plant A was 6.9 and Plant B was 7. Not only had the plants removed the acid rain that was added to them, but also the natural acid too. However, zero acid is not healthy for most plants. Plant nutrients such as manganese can become toxic if pH level is too low. Geranium plants are especially susceptible to this condition, displaying yellowed, brown-flecked, or dead leaves when growing in alkaline soils (Bonvissuto, n.d).
Figur 4.2: Picture of the plants before (right) and after (left) the experiment.
Throughout the experiment, the plants underwent a small amount of browning. The control plant had a pH of 7.2. The plant soil became slightly alkaline. This is caused by weathering, or the development of calcium carbonate-rich parent materials in arid or dry environments. Through this process, alkaline soils are formed. Acid-forming ingredients in soil amendments can temporarily reduce soil alkalinity; underlying rock types can permanently lower it. Alkaline plant materials increase soil pH over time. Due to the rapid evaporation of water in arid or desert conditions, soil becomes more alkaline over time, as salt builds up in the soil (Bonvissuto, n.d). The room in which the plants were kept was always locked, preventing air exchange. This caused the room to be dry. In addition, the soil was very dry because it was crumbly.
Figure 4.3: Week two results for the same plants after watering again.
I repeated the experiment again with the same plants. On February 28, 2022, I watered plants A and B again with acid rain and the control plant with tap water. The control plant’s pH decreased because of the tap water. In accordance with EPA guidelines, tap water should have a pH between 6.5 and 8.5 (Environmental Protection Agency, n.d). The water from the source I used was especially acidic. This caused the pH of the soil to decrease to 7.1. After watering with “acid rain”, the pHs of plants A and B were substantially lower than in the first week of the experiment (In the second trial, Plant A was 5.4 and Plant B was 5.3). This could have been caused by the previous “acid rain”. On March 2, 2022, I tested the soil pH again. Plant A’s was 6.9 and Plant B’s was 6.7. The control plant was slowly increasing in pH (7.3). The next day (March 3, 2022), both the pHs of plant A and the control plant stayed the same as the day before. Plant B’s increased to 6.9.
DISCUSSION
5.1 Sources of Error
One possible error could be caused by a person disturbing the experiment. I set up my experiment on top of a desk, in front of a bookshelf. A person who wanted to retrieve a book from the shelf could have knocked a plant or piece of equipment over and attempted to put it back.
Another possible source of error is an incorrect reading of the pH. The pH meter that I used did not have an exact decimal number. The meter only had whole numbers on it and the halfway marks were not always in the middle. Therefore, the experimental data could have been imprecisely collected.
The last possible error that could have occurred is the technique of measuring pH. At the beginning of my experiment, I measured the pH only in one spot within the soil. By the time I measured the second time I measured in many different spots until the data was constant. This could have made my initial and first measurement (February 22, 2022) inaccurate compared to the measurements in-between.
5.2 Next Steps
The next step is to repeat the experiment but eliminate the sources of error listed above. If the data is confirmed to be accurate, the experiment will be repeated but with different species of plants. Snake plants only thrive in rocky, dry habitats, mostly in Africa (New York Botanical Garden, n.d). However, acid rain is everywhere. Therefore, this experiment should be extended to other plants from different ecosystems. Sunflowers can be tested using a similar experimental setup. They will survive in North American plains and meadows (Boi, 2015). Native plants play an important role in maintaining the ecosystem by providing soil stability, filtration of water, cleansing of air, and support for native wildlife. Native plants that are adapted to their surroundings can significantly mitigate environmental stress, such as droughts and extreme temperatures, while preserving the productivity of farming and wildlife habitats. If plants that are not native to the ecosystem are planted, they could become an invasive species. Our natural environment and habitats are threatened by invasive species that can disrupt essential ecosystem functions. By competing for water, nutrients, and space, invasive plants displace native vegetation. By establishing and propagating themselves, invasive species can reduce soil productivity, impact water quality and quantity, threaten biodiversity, degrade range resources, alter natural fire regimes, and introduce diseases. By testing then planting only native species, we can learn how to mitigate the negative effects of acid rain on the soil without altering the ecosystem.
5.3 Applications
Snake plants could help solve a big environmental problem in today's world. Acid rain is polluting soil all over the world. Planting snake plants will remove acid rain from polluted soil without causing damage to the environment. There are other solutions to remove acid rain from soil, but most are inefficient and/or expensive. For instance, acid rain damaged soil can be repaired by calcium-based solutions. In New Hampshire, U.S.A, 40 tons of calcium pellets were dropped over a 29-acre watershed at Hubbard Brook using helicopters over several days (Landscape Water Conservation, 2020). By planting plants with phytoremediation properties, we can remove acid rain from highly acidic areas while still using our other solutions for large, less acidic, areas like they did in New Hampshire.
conclusion
In conclusion, Sansevieria trifasciata can remove acid rain from soil with little damage to the environment and in a short period of time. This study has many valuable implications to countries who suffer from the effect acid rain has on the environment and human health. If this study continues, countries around the world can use phytoremediation plants to remove acid from spots with a large pH quickly. The information from this study can solve the global crisis of acid rain in our soil. Once we have completed the study on different types of soil, snake plants, and climate we can solve the big question of “can Sansevieria trifasciata remove acid rain from soil”.
ACKNOWLEDGEMENTS
I would like to thank an amazing science teacher Mrs. Esch for her help with providing materials and helping me execute the experiment. I would also like to thank my friends Lana and Michayla for encouraging me all throughout the experiment and telling me constantly not to kill the plants. I would like to acknowledge Rashi’s help in answering my million questions. Lastly, I would like to thank Ameris, my mentor, for the help with editing.'
REFERENCES
19 types of snake plants (sansevieria varieties) with care and growing tips. Leafy Place. (2021, July 9). Retrieved March 4, 2022, from https://leafyplace.com/snake-plants-sansevieria-varieties/
6 snake plant benefits – plus guide to care and decor. Jay Scotts Collection. (2021, November 22). Retrieved March 4, 2022, from https://jayscotts.com/blog/benefits-of-a-snake-plant
Acid Sensitive Regions . (n.d.). Britannica . Retrieved from https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.britannica.com%2Fscience%2Facid-rain%2FEffects-on-lakes-and-rivers&psig=AOvVaw3Zew_gWYV85F7lCCfinNYh&ust=1647121849299000&source=images&cd=vfe&ved=0CAsQjRxqFwoTCID_s5mFv_YCFQAAAAAdAAAAABAD.
Boi, J. (2015, November 30). 5 best plants for phytoremediation. Land8. Retrieved March 5, 2022, from https://land8.com/5-best-plants-for-phytoremediation/
Bonvissuto, D. (n.d.). Alkaline water health benefits: Is alkaline water good for you? WebMD. Retrieved March 4, 2022, from https://www.webmd.com/diet/what-is-alkaline-water
Effects of Acid Rain. (n.d.). takacspomegranate. Retrieved from http://takacspomegranate.weebly.com/uploads/1/8/2/1/18212943/753444.gif?451.
Environmental Protection Agency. (n.d.). Ground-level Ozone Basics. EPA. Retrieved March 4, 2022, from https://www.epa.gov/ground-level-ozone-pollution/ground-level-ozone-basics
Environmental Protection Agency. (n.d.). What is Acid Rain? EPA. Retrieved March 4, 2022, from https://www.epa.gov/acidrain/what-acid-rain
Environmental Protection Agency. (n.d.). Why is Acid Rain Harmful? EPA. Retrieved March 4, 2022, from https://www3.epa.gov/acidrain/education/site_students/whyharmful.html
Canadian Council on Invasive Species. (n.d.). Retrieved March 11, 2022, from https://canadainvasives.ca/invasive-species/
Landscape-Water-Conservation. (2020, January 30). Solutions to soil problems: High ph. Water Conservation for Lawn and Landscape. Retrieved March 4, 2022, from https://landscape-water-conservation.extension.org/solutions-to-soil-problems-high-ph/#:~:text=Also%2C%20alkaline%20irrigation%20waters%20may,of%20the%20western%20United%20States.
McFadden, C. (2020, July 28). What acid rain is and ways to restore the damage it causes. Interesting Engineering. Retrieved March 5, 2022, from https://interestingengineering.com/what-acid-rain-is-and-ways-to-restore-the-damage-it-causes
Miracle-gro moisture control potting soil - 8.8 L. RONA. (2019, May 26). Retrieved March 4, 2022, from https://www.rona.ca/en/product/miracle-gro-moisture-control-potting-soil-88-l-741783-12675085
Natural Resources Conservation Service. NRCS. (n.d.). Retrieved March 11, 2022, from https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/home/?cid=STELPRDB1166100
Negri, Cristina & Hinchman, Ray. (1996). Plants That Remove Contaminants From the Environment. Laboratory Medicine. 27. 36-40. 10.1093/labmed/27.1.36.
Nunez, C. (2021, May 4). Acid rain. Environment. Retrieved March 4, 2022, from https://www.nationalgeographic.com/environment/article/acid-rain
Rupam. (2019, April 29). Best Indoor Air Purifying plants recommended by NASA snake plant/mother in law tongue : My Garden Forest. Retrieved March 4, 2022, from http://mygardenforest.com/best-indoor-air-purifying-plants-recommended-by-nasa-snake-plant-mother-in-law-tongue/
Schematic-representation-of-different-phytoremediation-approaches-by-plants-under-study. (n.d.). Research Gate. Retrieved from https://www.researchgate.net/publication/341531208/figure/fig1/AS:893442938916864@1590024804275/Schematic-representation-of-different-phytoremediation-approaches-by-plants-under-study.jpg.
Snake plant (sansevieria): Home. Research Guides. (n.d.). Retrieved March 5, 2022, from https://libguides.nybg.org/snakeplant#:~:text=Snake%20plants%2C%20also%20referred%20to,which%20are%20stiff%2C%20erect%20plants.
Software, pT. (n.d.). Biological agents. Health and Safety Authority. Retrieved March 4, 2022, from https://www.hsa.ie/eng/Topics/Biological_Agents/Biological_Agents_Introduction/What_are_Biological_Agents_/
The four things you need to know about soil ph. FineGardening. (2019, June 4). Retrieved March 4, 2022, from https://www.finegardening.com/article/the-four-things-you-need-to-know-about-soil-ph#:~:text=Too%20low%20a%20pH%20level,%2Dflecked%2C%20or%20dead%20leaves.Invasive species.
The pH Scale. (n.d.). EPA. Retrieved from https://www.epa.gov/sites/default/files/styles/medium/public/2016-03/500x350_phscale_3-2.png?VersionId=ahCfwgNJpzTXzWUJBUw_zz8Uz8AUswmK&itok=QoCEklrC.
Understanding different types of soil pollutants. Understanding The Different Types of Soil Pollutants. (n.d.). Retrieved March 4, 2022, from http://www.pvcplus.com/news/understanding-different-types-soil-pollutants.aspx
Watkins, D. (2020, November 17). Alkaline soil conditions. Home Guides | SF Gate. Retrieved March 4, 2022, from https://homeguides.sfgate.com/alkaline-soil-conditions-76378.html
What makes soil alkaline – plants ... - gardening know how. GardeningKnowHow. (n.d.). Retrieved March 4, 2022, from https://www.gardeningknowhow.com/garden-how-to/soil-fertilizers/alkaline-soil-plants.htm
ABOUT THE AUTHOR
Natalia Papaj
Natalia is a grade 10 student attending Holy Cross in Strathroy, Ontario. She enjoys participating in scientific research and athletics. She intends to take part in future science and journalistic competitions. Natalia enjoys writing poetry, short stories, essays, and journals in her free time. She is interested in software engineering as a future career. Natalia loves learning about today’s environment to create a better future for the next generation.