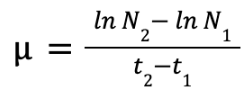Alpita Patro
she/her | Age 14 | St. John’s, NL
1st place in Junior Life Sciences, 2nd place overall at provincial level | Sanofi BioGENEius Award | Canada-Wide Science Fair 2022 bronze medal
Edited by Vanessa Poirier
Burning fossil fuels has affected Earth’s ecosystems dramatically in recent years. Canada alone burned 1,309 gigawatt-hours of fossil fuels in 2017, which increased to 1,516 gigawatt-hours in 2018 (Statistics Canada, 2021). This intense consumption stresses the importance of conducting research on viable alternatives to fossil fuels. Biofuel is a source of renewable and sustainable energy that is derived from living matter such as animal waste, plants, fungi, and algae. In this experiment, four different species of algae (Tetraselmis sp., Pavlova sp., Isochrysis sp., and Chaetoceros sp.) were cultivated in laboratory conditions over fourteen days. A light meter application (Lux Light Meter Pro) was used to measure the cell count for each culture. Results from this experiment showed that Pavlova sp. had the most significant increase in cell count (91, 000 cells) and decrease in light intensity (14 lux), yet Tetraselmis sp. maintained the highest growth rate of 5.1%. Therefore, Tetraselmis sp. would acquire the most biofuel due to its high lipid content. Research like this is fundamental because the knowledge of the extent to which species-specific algae produce the most biofuel will help make biofuel production economically feasible and efficient.
INTRODUCTION
In recent years, drastic changes in weather patterns, such as an increase in snowstorms, hurricanes, and excessive rainfall have been noticed in many areas, including Newfoundland and Labrador. These dramatic alterations are most likely caused by global climate change (Williams, 2019). One of the leading causes of global climate change is the building up of greenhouse gasses in the atmosphere due to the burning of fossil fuels (The Causes of Climate Change, 2022). Canada alone burned 1,309 gigawatt-hours of fossil fuels in 2017, which increased to 1,516 gigawatt-hours in 2018 (Statistics Canada, 2021). These findings in fossil fuel propelled me to conduct research on biofuel, which is considered an alternative to fossil fuel.
Newfoundland and Labrador is surrounded by vast seawater, and the population of algae in its water is astronomical. My curiosity grew: could algae be a prime source of biofuel? After much research and inquiring to professionals, I found that fast growing microalga species produce large amounts of biomass. I also learned that algal lipid content is crucial for biofuel production, as it has fatty acids. I ventured out to cultivate four different species of algae (Isochrysis sp., Tetraselmis sp., Chaetoceros sp., and Pavlova sp.). By doing so, I wish to find which species possess the fastest growth rate, attain the highest lipid content, and produce the most biofuel.
HYPOTHESIS
Three hypotheses were tested by carrying out the above experiment: (1) Tetraselmis sp. would have the highest growth rate because of the predominance of chlorophyll. (2) Tetraselmis sp. should produce the most biofuel because these species acquire a large amount of fatty acid, specifically docosahexaenoic acid (DHA). (3) The light intensity of all algae cultures will decrease as algae biomass increases because the algal cells will absorb light from the fluorescent light source.
MATERIALS & METHODS
During the experiment, each of the four species was cultivated in separate containers for fourteen days. The containers were filled with 2 liters (L) of 30 parts per thousands (ppt) salt water kept at room temperature and 3 milliliters (mL) of F/2 nutrient solution parts A and B. Three species (Tetraselmis sp., Pavlova sp., and Chaetoceros sp.) were connected to a fish tank air pump to aerate the culture. I intentionally let Isochrysis sp. grow without aeration to see if this affects the growth rate. The Chaetoceros sp. required an additional 20 mL of silicate along with the F/2 nutrient solution in order to grow. Each culture was kept approximately 20.3 centimeters (cm) away from the fluorescent light.
A Llight Mmeter application (Lux Light Meter Pro) was used to measure the light intensity which was then used to measure the growth rate of algae. The application downloaded on my iPhone XR was placed 30 cm away from each culture, and the light intensity was measured for fourteen consecutive days. On the last three days of my experiment, the cell count of each species was conducted using a microscope. Then, 2 mL of each species was filtered using coffee filter paper. The species was then transported to a microscope, where the cells were manually counted.
Due to the toxicity of chemicals required for oil extraction, I could not perform the following portion of the experiment. However, I carried out a literature review on extracting biofuel from algae. Once the algae were grown, each species could be filtered and left for several hours to dry. A ratio of 20 mL of chemical solvent hexane to 1 gram (g) of dry-weight algae can be used to extract oil from algae. It would then take a few hours until the oil entirely separates. Once it is complete, the algae have sunk to the bottom, and the oil is afloat. A graduated syringe can extract and measure how much oil is present in each culture. The species that accumulates the most oil will also produce the most biofuel (Hannon et al., 2010).
RESULTS
The findings from this experiment express remarkable outcomes. As shown in Figures 1.1 and 1.2, the color of all algae species changed dramatically throughout all fourteen days. On Day 1 (Figure 1.1), it is evident that each culture looked lightly tinted. As the days went by, the color of each culture became more prominent.
Figure 1.1: Cultures on Day 1. From left to right: Pavlova, Tetraselmis, Chaetoceros, and Isochrysis.
Figure 1.2: Cultures on Day 14. From left to right: Pavlova, Tetraselmis, Chaetoceros, and Isochrysis.
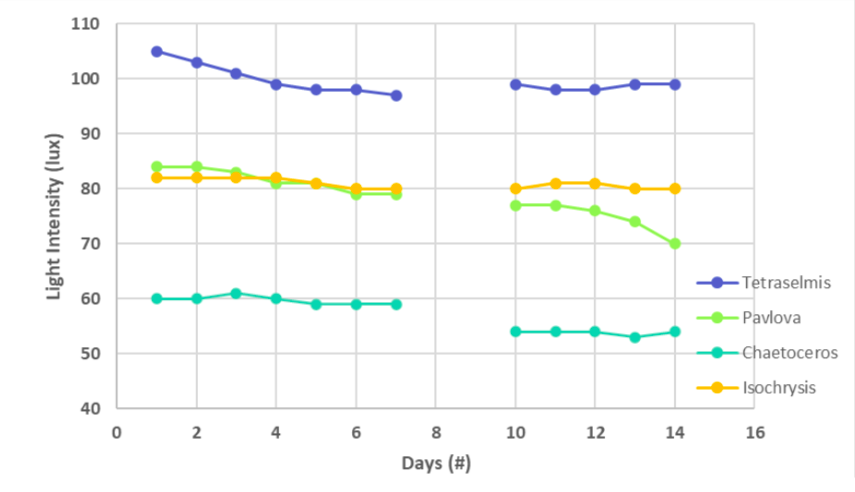
Figure 2 shows that as the turbidity of the cultures increased and the light intensity decreased. A linear regression equation between the cell count and corresponding light intensity was developed, as shown in Figure 3. In the equation, y represents the cell count while x represents the light intensity in lux. It appears that the cell count is indirectly proportional to the light intensity. Therefore, the regression equation was used to calculate the cell count of all species.
Figure 2: Light intensity versus days.
Figure 3: Light intensity versus cell count.
Figure 4 shows the cell count of all species during the fourteen days. Two data points were missed in Figures 3 and 4 during days 8 and 9. The absence of these data occurred because a light bulb decamped as the algae species grew. Surprisingly, the Pavlova species had the most significant decrease in light intensity of 14 lux (Figure 3), coinciding with an increase of 91, 000 cells (Figure 4). Tetraselmis sp., Chaetoceros sp., and Isochrysis sp. increased by 54,000; 55,000; and 18,000 cells, respectively. The light intensity decreased by 6 lux for Tetraselmis sp. and Chaetoceros sp., and 2 lux for Isochrysis sp.
Figure 4: Cell count versus days.
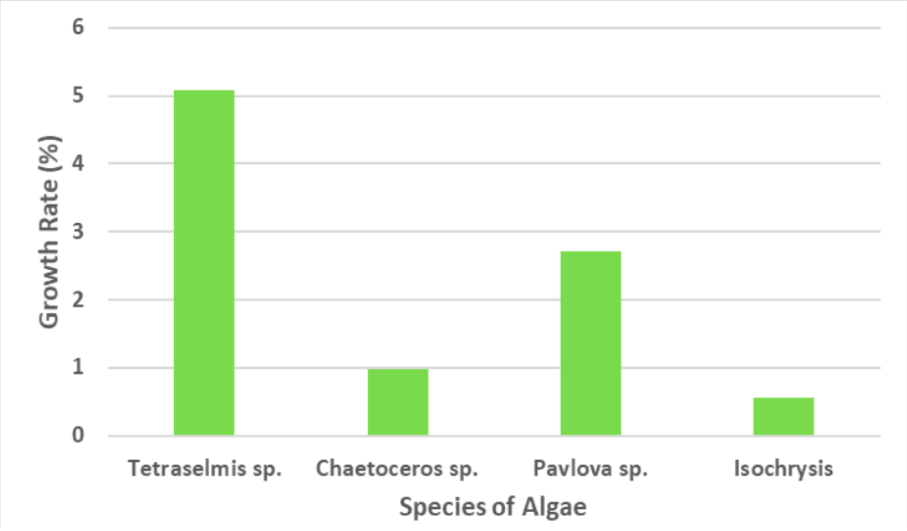
Figure 5: Growth rate in %
Levasseur et al. (1993) calculated the growth rate of marine phytoplankton using the following equation:
where μ = growth rate in percentage ; N1 = biomass at time1 (t1) ; N2 = biomass at time2 (t2)
The growth rate in percentage was calculated using the following equation:
The growth rate equation (Equation 2) was used to determine the growth rate of each algae species, shown in Figure 5. Tetraselmis sp. had the highest growth rate of 5.1% while Pavlova sp., Chaetoceros sp., and Isochrysis sp. had a growth rate of 2.7%, 1%, and 0.6%, respectively.
DISCUSSION
Figures 1.1 and 1.2 show that the color of all cultures changed from transparent to pigmented throughout all fourteen days. This change in pigmentation suggests that color is a good indicator of whether the species are growing but does not determine the growth rate of algae.
The light source was constant throughout the experiment and the light meter application was used to measure light intensity of each culture. Figure 2 shows that as the turbidity of the cultures increased, the light intensity decreased. A high concentration of algae absorbs more light from the fluorescent light source and reflect less (Metsoviti et al., 2019). As a result, the light intensity decreased.
Figure 4 displays the cell count of all species during the experiment. Pavlova sp. had the most significant decrease in light intensity (Figure 3), coinciding with the increased cell count (Figure 4). Pavlova sp. has a symbiotic relation with bacteria strains such as Citrobacter sp. These bacteria strains enhance growth promotion and co-culturing by synergistically effecting each other’s metabolism (Ahamed et al., 2015). Therefore, Pavlova sp. may have had a significant decrease in light intensity and increase in cell count due to the hyperproliferation of Citrobacter sp.
Based on Equation 2, results show that Tetraselmis sp. had the highest growth rate of 5.1%, thus supporting my hypothesis. This difference occurred because the excessive chlorophyll in Tetraselmis sp. stimulates algae growth (Pereira et al., 2019). Pavlova sp., Chaetoceros sp., and Isochrysis sp. had a growth rate of 2.7%, 1%, and 0.6% respectively. The Isochrysis species’ cell count did not vary significantly. This invariant cell count proves that aeration is essential in growing algae. Chaetoceros sp. had the second-lowest growth rate, suggesting that the difference in nutrients did not affect the growth rate of algae.
Biofuel production is economical and easy due to its very few requirements (Gharagozloo et al., 2014). When algae grow, they undergo photosynthesis, converting sunlight energy into energy stored in oils, more specifically known aslipids. The lipids form triacylglycerols, which can be separated from algae through Hexane Soxhlet extraction. (Cassidy, 2010). The mixture then goes through the next phase: transesterification. This process allows chemical catalysts like methanol to react with the hexane solution and create a mixture of glycerol and biodiesel (Algae to Energy Systems Lab Experiments in Growth Optimization, 2015). Lastly, in the decatenation stage, the glycerol and biodiesel mixture will separate in resting tanks due to gravity and different densities. It is estimated to take 8-24 hours until the process is complete. Once it is finished, biodiesel will float, leaving glycerol to sink to the bottom of the tank (Bulnes et al., 2021). The left-over glycerol is not wasted, instead, the mixture is purified through acidification. The glycerol is used for food supplements or as humectant in pharmaceutical formulas (Abdul Raman, 2019). Based on my literature review, Tetraselmis sp. acquires the most lipids because of the dominance of Docosahexaenoic Acid (DHA) and other fatty acids, which are critical metabolic precursors for lipid mediators (Harwood, 2019). As a result, the Tetraselmis species will produce the most biofuel.
Biofuel is a form of renewable energy (Biofuel, 2021). It is mainly used as transportation fuels, but as research evolves, biofuel may be used to generate electricity and heat. It is important to explore different sources of energy to see which can help make planet Earth sustainable.
CONCLUSIONS
Findings from my experiment can be summarized as follows: (1) Tetraselmis sp. has the highest growth rate among all four species and Pavlova sp. endured the most significant change in light intensity and cell count. (2) Isochrysis sp. had the lowest cell count and growth rate, showing that aeration plays a critical role in the conservation of algae. (3) Tetraselmis sp. has obtained the highest oil content because of the supremacy of DHA. Though many exciting outcomes have been obtained through this experiment, much more research needs to be carried out such as finding what other species will acquire the most biofuel, if there are other harmless methods of obtaining biofuel from algae, the circumstances needed for optimal algae growth, etc. Growing algae on a commercial scale may also be beneficial because the oil will be extracted in larger volumes, making biofuel production economical and feasible.
ACKNOWLEDGEMENTS
I would like to thank Mersheen Bay Oysters and Memorial University for providing me with algae samples used in this experiement, as well as the College of the North Atlantic and Macdonald Drive Junior High School for giving me the materials needed for this project. A special thanks goes to my science teachers and parents for mentoring me.
REFERENCES
Abdul Raman, A. A., Tan H. W., & Buthiyappan, A. (2019). Two-Step Purification of Glycerol as Value Added by Product from the Biodiesel Production Process. Front. Chem. 7,774. https://doi.10.3389/fchem.2019.00774
Ahamed, S. A. K., Kim, J.-J., Chio, T.-O., & Choi, T.-J. (2015). Growth Promotion of Pavlova Viridis by Bacteria Isolated from the Microalgae. Journal of Life Science, 25(5), 568-576. Retrieved April 21, 2022, from https://www.researchgate.net/publication/282061751_Growth_Promotion_of_Pavlova_viridis_by_Bacteria_Isolated_from_the_Microalga
Algae to Energy Systems Lab Experiments and Growth Optimization (2015). Retrieved April 21, 2022 from https://btiscience.org/wp-content/uploads/2015/12/b.-Algae-to-Energy-Teacher-Manual-2015.pdf" https://btiscience.org/wp-content/uploads/2015/12/b.-Algae-to-Energy-Teacher-Manual-2015.pdf
Biofuel (2021). Encyclopedia Britannica. Retrieved April 19, 2022, from https://www.britannica.com/technology/biofuel
Bulnes, K., Paredes, D., & Vinces, L. (2021). An automatic biodiesel decanting system for the optimization of glycerin separation time by applying electric field and temperature. In Iano, Y., Arthur, R., Saotome, O., Kemper, G., Borges Monteiro, A.C. (Eds.), Proceedings of the 5th Brazilian Technology Symposium (pp. 349–355). Springer. https://doi.org/10.1007/978-3-030-57566-3_34
Cassidy, S. (2010, December 27). How can algae be converted into biofuel? HowStuffWorks. https://auto.howstuffworks.com/fuel-efficiency/biofuels/convert-algae-to-biofuel.htm
Gharagozloo, P. E., Drewry, J. L., Collins, A. M., Dempster, T. A., Choi, C. Y., & James, S. C. (2014). Analysis and modeling of Nannochloropsis growth in lab, greenhouse, and raceway experiments. Journal of Applied Phycology, 26(6), 2303–2314. https://doi.org/10.1007/s10811-014-0257-y
Statistics Canada. (2021, December 13). Electricity generated from fossil fuels, annual. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=2510002801
Hannon, M., Gimpel, J., Tran, M., Rasala, B., & Mayfield, S. (2010). Biofuels from algae: challenges and potential. Biofuels, 1(5), 763–784. https://doi.org/10.4155/bfs.10.44
Harwood, J. L. (2019). Algae: Critical Sources of Very Long-Chain Polyunsaturated Fatty Acids. Biomolecules, 9(11), 708. https://doi.org/10.3390/biom9110708
Levasseur, M., Thompson, P. A., & Harrison, P. J. (1993). Physiological Acclimation of Marine Phytoplankton to Different Nitrogen Sources1. Journal of Phycology, 29(5), 587–595. https://doi.org/10.1111/j.0022-3646.1993.00587.x
Metsoviti, M. N., Papapolymerou, G., Karapanagiotidis, I. T., & Katsoulas, N. (2019). Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants, 9(1). https://doi.org/10.3390/plants9010031
National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 8058, Hexane. https://pubchem.ncbi.nlm.nih.gov/compound/Hexane"
Pereira, H., Silva, J., Santos, T., Gangadhar, K. N., Raposo, A., Nunes, C., Coimbra, M. A., Gouveia, L., Barreira, L., & Varela, J. (2019). Nutritional Potential and Toxicological Evaluation of Tetraselmis sp. CTP4 Microalga Biomass Produced in Industrial Photobioreactors. Molecules, 24(17), 3192. https://doi.org/10.3390/molecules24173192 NASA. (2022). The Causes of Climate Change. https://climate.nasa.gov/causes/
Williams, H. S., (2019). Climate Change, Natural Disasters, and Wildlife. National Wildlife Federation. https://www.nwf.org/-/media/Documents/PDFs/Environmental-Threats/Climate-Change-Natural-Disasters-fact-sheet.ashx
ABOUT THE AUTHOR
Alpita Patro
Alpita Patro is a grade 9 student attending Macdonald Drive Junior High School in St. John's, Newfoundland and Labrador. She has a penchant for science, mathematics, and language arts, as she is actively involved in STEM clubs, math leagues, public speak-offs, and spelling bees. Aside from academics, Alpita enjoys swimming, playing badminton, dancing, and playing the piano. In the future, she wishes to pursue a career in medicine. After noticing drastic changes in weather patterns, Alpita was determined to start a project that can significantly contribute to contending environmental struggles. She utilized multifunctional products like algae to produce a sustainable energy source like biofuel. With this project, Alpita hopes to bring awareness to Climate Change.