SARAH MCDONALD
she/her | age 17 | Vancouver Island, BC
Edited by Talia Rossi & Shannon MacDonnell
INTRODUCTION
Many studies have quantified the effects of cigarettes and vapes on the human body (Smith, 2008), however, cigarettes’ environmental effects have only beenbriefly explored and the impact of vapes remains unstudied (World Health Organization, 2017). This study aims to evaluate the impact of vaping juice and cigarette butts on the chemical composition of lake water and the potential hazards imposed on lake water ecosystems. In doing this, this study seeks to determine the effects on humans through the extrapolation of collected data. This is important as drinking water tainted with chemicals and heavy metals can result in disastrous public health consequences (USA Today, 2017; Smith, 2008; World Health Organization, 2017).
E-cigarette juice and cigarette butts were introduced to lake water samples from two lakes in the Cowichan Valley: Shawnigan Lake and Fuller Lake. After two weeks, their chemical compositions were analyzed using mass spectrometry and compared to control samples. Changes were measured through quantified differences in concentrations of various elements relative to the control group by using inductively coupled plasma mass spectrometry (ICP-MS). Arsenic, nickel, lead, tin, cadmium, and manganese were chosen for analysis for distinct reasons relating to their prevalence in cigarettes and vape juice, as well as their known detrimental effects on both humans and the environment (“USA Today, 2017; Smith, 2008; World Health Organization, 2017).
METHODS
Five 1.0 L samples were collected in High density polyethylene (HDPE) bottles from each of the aforementioned locations. HDPE containers were used to prevent sample contamination, and powder-free latex gloves were also worn during sample collection. These samples of 1.0 L, of which aliquots were used for experimentation, were all taken from a central point of each lake at a depth of 45 cm to reduce the likelihood of contamination (Prestige Pools and Spas, n.d.). These samples were then divided into 10 x 250 mL aliquots which were then used to complete experimental procedures. Lake water samples were placed in 500 mL HDPE bottles and the isolated contaminants were introduced to their respective samples by being dropped or poured into the bottles. A third of the samples from each lake were set aside to use as controls, a third of the samples had cigarette butts introduced to them, and the remaining third had e-cigarette juice added to them.
Before adding the cigarette butts, they were prepared in a manner that mimicked the process a cigarette undergoes when being smoked. Cigarettes were lit and then attached to a garden-hose nozzle which then created a vacuum and drew the smoke through the cigarettes and left the butts behind. It was these butts which were then introduced to a third of the samples from each lake. One cigarette butt was added to each of these samples. Five millilitres of E-juice was measured and introduced to the “vape” samples, as that is approximately how much e-juice is contained within a single vape (Canada Vapes, n.d.).
Samples were left for two weeks. After two weeks had passed, samples were analyzed at the University of Victoria’s Geochemistry and ICP-MS lab. The concentrations of elements inthe contaminated trials were measured against that of control groups. A summary of the analysis procedure has been provided below.
Day 1
Disposable plastic spatulas were used to remove the cigarette butts from the water samples, andthe cigarette butts were set aside to later be disposed of in a safe manner. All samples were thenacidified to approximately 1.5 % HNO3 using 2 mL of 16 Molar Anachemia Environmental Grade HNO3 per 100 mL of sample water. The acid was added directly to the sample bottles, and after introduction, lids were placed back on the samples and the solutions were mixed well. They were then left to equilibrate for approximately 1 hour. Bottles were shaken several times during this period.
While samples were equilibrating, sample vials were prepared. Samples vials were labeled and then rinsed three times using deionized water. Samples were then pipetted into the vials. The first volume of approximately 6 mL was used to rinse the vial, and rinsings were collected to make a drift solution. After rinsing, solutions of around 6 mL were then pipetted into the vials. Batches of 2 samples were then centrifuged at 2500 rpm for 10 minutes to protect the ICP-MS from any particulates in the samples which could plug the nebulizer. Samples were then loaded into the autosampler rack, and an autosampler list was made.
Day 2
The ICP-MS was set up for solution analysis and then started up. The required setup and performance testing routines were completed. The analysis batch details were then entered (i.e., sample list, element list, run and acquisition conditions), after which the batch was run and data was collected.
After the data was collected, a statistical analysis was performed. For the data reflective of the controlled samples of each element, the mean and standard deviation were calculated and combined to reflect both values as represented in the expression: mean ± standard deviation. For the data reflective of the respective cigarette butt and e-juice contaminants, averages and standard deviations were calculated and then compared with the control data to determine P-values for each data set.
RESULTS
In every statistically viable data set — which was defined as a data set with a computed P value of less than 0.05 therefore suggesting a 95% confidence level — exposure to cigarette butts and e-juice were shown to elevate the concentration of every element chosen for analysis with percentile increases ranging from ~ 11 % to ~ 6500 %. It should be noted that while all statistically viable data sets met the criteria of having a P-value of less than 0.05, 75 % of these data sets had a P-value of < 0.00001.
Elemental concentration graphs are displayed on the following pages. Columns with black dashed borders indicate a lack of statistical significance due to a computed P-value of greater than 0.05.
Figure 1: Average Arsenic Concentrations in Lake Water After Exposure to Contaminants (μg/L)
Figure 2: Average Nickel Concentrations in Lake Water After Exposure to Contaminants (μg/L)
Figure 3: Average Lead Concentrations in Lake Water After Exposure to Contaminants (μg/L)
FIgure 4: Average Tin Concentrations in Lake Water After Exposure to Contaminants (μg/L)
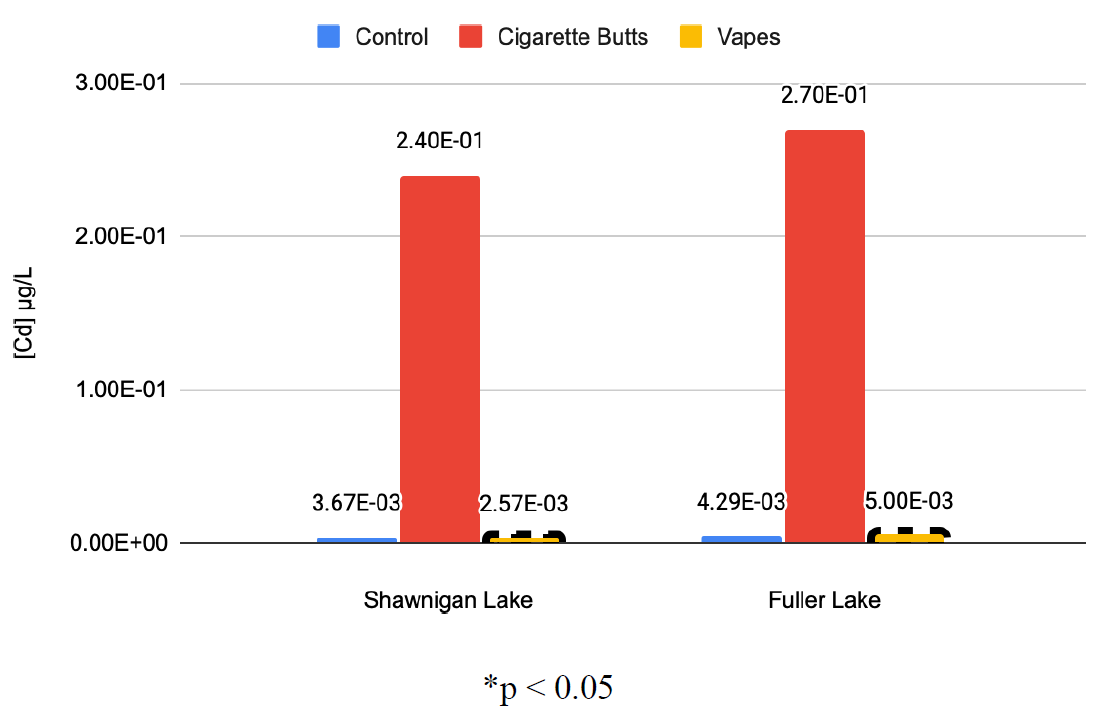
Figure 5: Average Cadmium Concentrations in Lake Water After Exposure to Contaminants (μg/L)
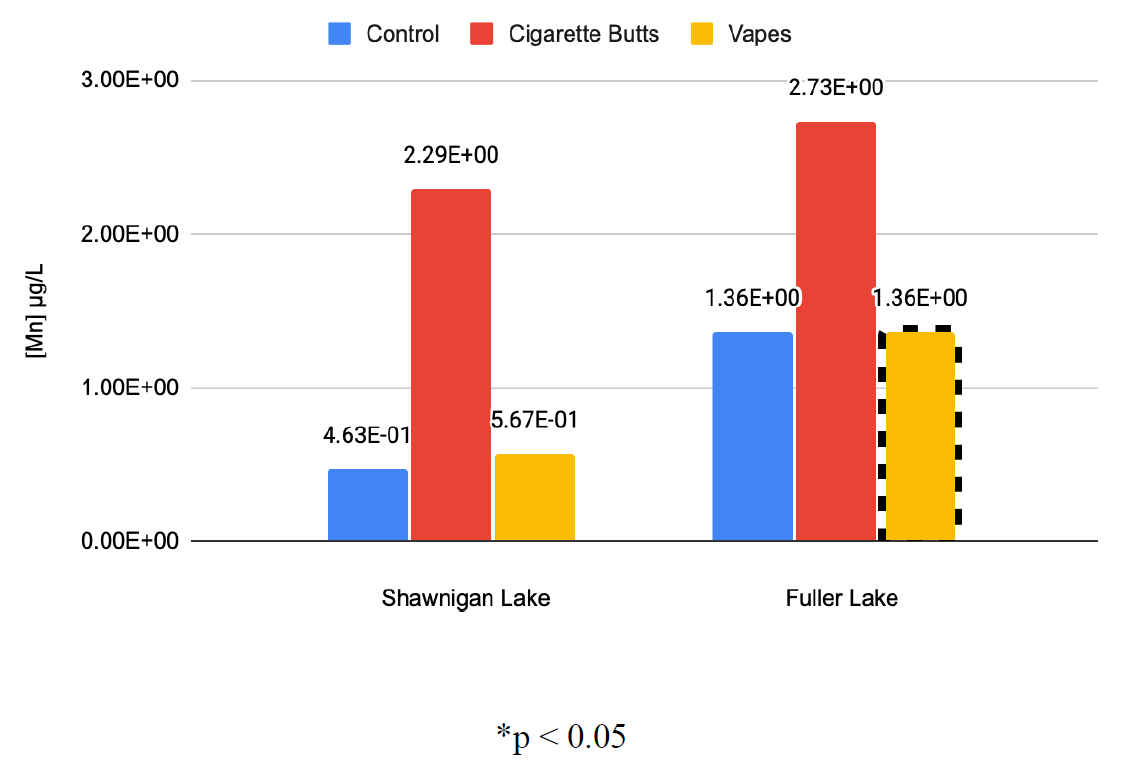
Figure 6: Average Manganese Concentrations in Lake Water After Exposure to Contaminants (μg/L)
DISCUSSION
Results showed that exposure to cigarette butts consistently increased the concentrations of the assessed elements within samples which were consistently of significant magnitudes thus illustrating their detrimental impact on aqueous ecosystems. Introduction of vaping e-juice to water samples showed similar results. All significant results showed increases in elemental concentration, however unlike the data collected from cigarette butt contamination, not all results were deemed statistically significant.
It should also be noted that in all studied cases except for arsenic, results showed that exposure to cigarette butts resulted in far higher elemental concentrations; the average percentage increase in elemental concentration across all cigarette butt datasets was 1378 % that of the controlled samples. While exposure to vaping e-juice also showed increased concentrations, they were far less drastic, with the average percentage increase in elemental concentration across all e-juice datasets being 156% that of the controlled samples. This allows us to determine that while both cigarette butts and e-juice are detrimental to the environment, cigarette butts have a significantly larger impact on aquatic ecosystems.
These results allow for a clear comparison between cigarette butts and vaping e-juice as contaminants. With arsenic as the only exception, all studied elements showed higher levels after exposure to cigarette butts as compared to exposure from vaping e-juice. We can then infer that cigarette butts pose a larger environmental threat than vaping e-juice, as they release more toxic compounds per unit. Further research on the environmental effects of cigarette butts and vaping e juice should focus on concerns of bioaccumulation, which need to be further studied to fully understand environmental impacts (Hoke et al., 2016).
CONCLUSION
While the detrimental effects of cigarettes on the human body are widely known, the environmental effects of both cigarettes and vapes have been vastly understudied despite their prevalence in our society. By testing the effects of cigarette butt and vaping e-juice exposure on lake water samples, this study established that exposure to vaping e-juice and cigarette butts results in increased concentrations of arsenic, nickel, lead, tin, cadmium, and manganese in lake water. Contaminated samples contained higher concentrations of several known carcinogens and toxic heavy metals compared to controls, with percentage increases reaching as high as 6500 %. This study confirmed the hypothesis that both cigarette butts and e-juice are detrimental to the environment and determined that cigarette butts are significantly more harmful to aquatic ecosystems than vaping e-juice overall.
ACKNOWLEDGEMENTS
I wish to extend my special thanks to Mr. Justin Wilke and the Science department at Shawnigan Lake School for their unwavering support of this project, as well as the Geochemistry Department at the University of Victoria for the use of their facilities. I would also like to express my most sincere gratitude to Dr. Jody Spence for his guidance and expertise, as well as Yeon Jae Lee (NYU Abu Dhabi ‘24) for supporting me throughout the project and providing valuable insight into the study.
REFERENCES
Hazardous chemicals discovered in vapor.(Brief article). (2017, Feb 1,). USA Today (New York, N.Y.), 145, 16. https://search.proquest.com/docview/1863551602
Hoke, R., Huggett, D., Brasfield, S., Brown, B., Embry, M., Fairbrother, A., Kivi, M., Paumen, M. L., Prosser, R., Salvito, D., & Scroggins, R. (2016). Review of laboratory-based terrestrial bioaccumulation assessment approaches for organic chemicals: Current status and future possibilities. Society of Environmental, 12(1),109-122. https://10.1002/ieam.1692
How much e-liquid will I need? Canada Vapes. Retrieved Mar 19, 2021, fromhttps://canadavapes.com/info/much-e-liquid-will-need.html
How to Properly Take a Water Sample. Prestige Pools and Spas. Retrieved Mar 17, 2021, from https://www.prestigepoolsandspas.com/blog/how-to-properly-take-a-water-sample-for-t esting
Smith, S. (2008, Nov 1,). Chemical chaos: many people have only a basic idea of why tobacco smoke is unhealthy. Read on to find out even more reasons not to light up. Scholastic Choices, 24, 17.
World Health Organization. (2017). Tobacco and its environmental impact: an overview. https://escholarship.org/uc/item/8tp3r5rc
ABOUT THE AUTHOR
Sarah McDonald
Sarah McDonald completed this experiment while attending high school at Shawnigan Lake School, on Vancouver Island, BC, Canada. She has always been interested in human biology, and is hoping to pursue a career relating to her interests in immunology, nanotechnology and bioengineering after finishing her studies at McGill University. In her spare time she loves hiking, reading, and is an avid smoothie drinker.