Haarini Suntharalingam
Age 16 | Markham, Ontario
York Region Science and Technology Fair 2019 Silver Medal Winner, Youth Science Canada Online Stem Fair 2020 YSC Disease and Illness Challenge Ribbon Winner, National Ribbon Winner and Regional Ribbon Winner
Melanoma is the deadliest form of skin cancer affecting the melanin in skin cells. However, if it is detected early, there are high chances of recovery as the 5-year survival rate has increased drastically throughout the past few years. Typically, melanoma is detected by utilizing the ABCDE's of melanoma detection (asymmetry, border, colour, diameter, and evolving) (Centers for Disease Control and Prevention – Division of Cancer Prevention and Control, 2020) with the aid of a dermatologist. However, it cannot be 100% confirmed whether the lesion is cancerous or not without a biopsy in which melanoma can metastasize to other parts of the body before even knowing it exists within.
This article consists of a systematic review and a proposal for an innovation that could revolutionize the method of diagnosis of melanoma. The systematic review component will explore the potential relationship of the presence of lipofuscin in melanoma, and the innovation section will propose a novel diagnostic method for this ailment. As a whole, this article will propose a potential biomarker that can be used to diagnose melanoma along with the development of a detection device that could be used to diagnose melanoma utilizing this biomarker.
INTRODUCTION
Melanoma is a form of skin cancer caused by the rapid division of melanocytes, developing in the deeper layers of the epidermis (Canadian Cancer Society, n.d.). The primary cause of melanoma is ultraviolet (UV) radiation penetration in the skin since it causes mutations in healthy melanocytes, thus inducing the rapid division of these cells. UV radiation induces oxidative stress in cells, and studies suggest that this can induce the accumulation of lipofuscin within cells (Di Guardo, 2015). Not only does lipofuscin have relevance to the pathogenesis of melanoma, but it is also a biomarker of choroidal melanoma. Further, this pigment has been observed in malignant and benign forms of pancreatic cancer, non-choroidal melanoma (Jegou Penouil et al., 2014) and mammary gland carcinoma; thus it could be a potential biomarker for melanoma.
Lipofuscin is an autofluorescent pigment which contains lipid residues - a byproduct of cell digestion stored in the lysosome of cells. It is typically a sign of ageing cells since its accumulation is induced by oxidative stress. The unique autofluorescent quality of lipofuscin makes it a potentially effective biomarker for the imaging of cancerous cells. Lipofuscin has not yet been targeted as a biomarker for melanoma, nor for any form of cancer as it has not been investigated much in the past. Hence, this article will analyze the existing literature on this topic.
This article aims to identify whether lipofuscin is present in melanoma to confirm whether it could be distinguished as a melanoma biomarker and to develop a diagnostic device for imaging lipofuscin presence.
METHODS AND DESIGN
This article was written by analyzing a study on the formation of lipofuscin in Alzheimer’s Disease (de Melo et al., 2013), which has been associated with the possible formation of lipofuscin in melanoma. The analysis of factors that support the presence of lipofuscin in melanoma will be presented in the Results section. In this study, various mechanisms that contribute to the formation of lipofuscin have been analyzed. As senescence is a significant contributor, the relationship between cancer and neurodegenerative diseases was researched since lipofuscin is present in these groups of diseases.
The following assay would have been completed if access to necessary resources and mentorship was provided:
This article was written by analyzing a study on the formation of lipofuscin in Alzheimer’s Disease (de Melo et al., 2013), which has been associated with the possible formation of lipofuscin in melanoma. The analysis of factors that support the presence of lipofuscin in melanoma will be presented in the Results section. In this study, various mechanisms that contribute to the formation of lipofuscin have been analyzed. As senescence is a significant contributor, the relationship between cancer and neurodegenerative diseases was researched since lipofuscin is present in these groups of diseases.
The following assay would have been completed if access to necessary resources and mentorship was provided:
Variables
The variables are the different forms of cells, both neoplastic melanoma cells and healthy skin cells. The simplest experimental method for this project consists of using healthy skin cells as the negative control group and comparing it to a variable, perhaps cell from the common B16 melanoma cell line.
A refined approach would alter the previously stated method to have multiple variable groups with cell lines from different types of melanoma (superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, choroidal melanoma and acral lentiginous melanoma). These variable groups would be compared to the control group of healthy cells.
Appendices
A Sudan Black B assay can be completed to determine the presence of lipofuscin in melanoma cells. (Georgakopoulou et al., 2013). The Sudan Black B histochemical stain is lipophilic in such a way that if lipofuscin is tested positive in a cell, a visual representation of it would be identified as Sudan Black B is a stain that exclusively targets the lipofuscin present in a cell.
Method: Susan Black B Assay
With the assumption that the cell lines are already prepared, the following steps will be followed:
Cells will be pipetted into microplates in which cell media will be added into each of the wells at a specific ratio.
With the correct proportions, the Sudan Black B histochemical stain will be added to all wells.
Cells will then be cultured for 72-96 hours at a temperature of 37°C with 5% carbon dioxide in an incubator.
Cells can then be analyzed under a compound microscope to visualize whether lipofuscin is present in the cells or not through concluding whether dark blue-black granules can be observed.
Innovation Design
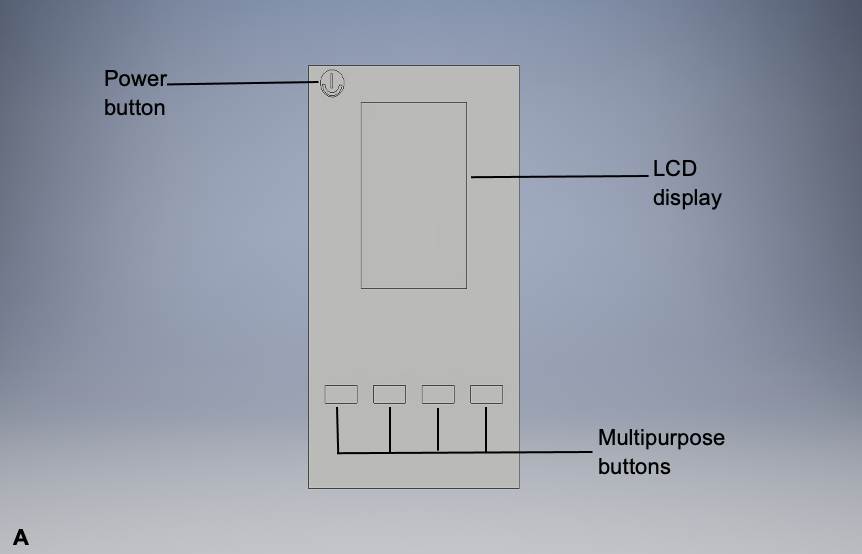
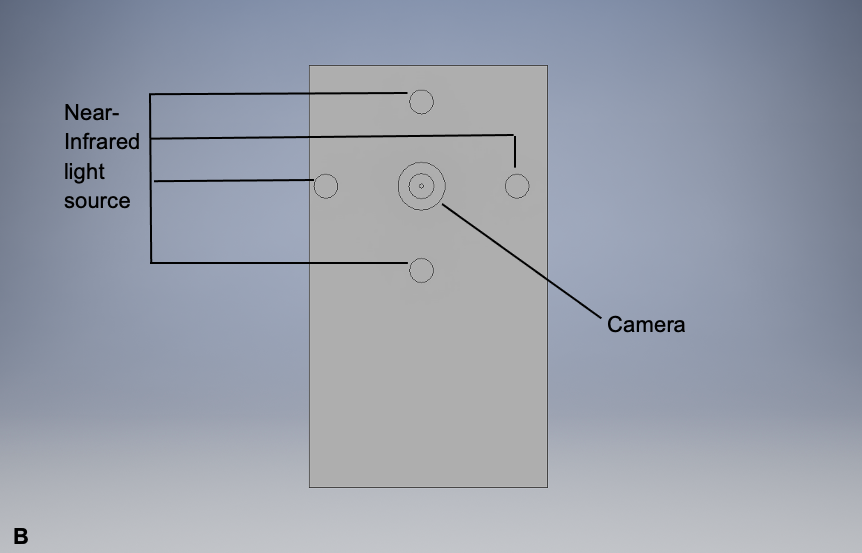
As previously mentioned in the Background Information section, lipofuscin has autofluorescent properties, allowing it to be visualized via autofluorescent imaging techniques. This potential imaging tool would be similar to the fundus autofluorescence imaging technique (Mercado, 2015), which is currently being used to image choroidal melanoma since this is already used to visualize lipofuscin levels in the eye. However, it would be a handheld device similar to the PRODIGI (Portable Real-time Optical Detection Identification and Guide for Intervention) autofluorescence imaging device (DaCosta et al., 2015), which is currently being used to visualize wounds. This handheld device would visualize lipofuscin by emitting excitation wavelengths ranging from 400-700 nm, where lipofuscin fluorophores would emit 500-700 nm as a result. These wavelength values are simply estimates based on existing research (Wu et al., 2014). A prototype design of this device and its sections relevant to its effectiveness are represented in Figure 1.
Note: This device has an LCD display that would demonstrate the presence of lipofuscin in a melanoma lesion by using its camera. The lipofuscin would fluoresce as a result of the near-infrared light source on this potential device. The multipurpose buttons would enhance the examiner’s view of the melanoma lesion.
RESULTS
Connection between lipofuscin and melanoma
Although many factors are associated with melanoma, the leading cause of melanoma is exposure to UV radiation (Mayo Clinic, 2020). UV radiation causes oxidative stress (Santacruz-Perez et al., 2018)on cells as this form of radiation releases inflammatory cytokines (Goswami et al., 2013). An inflammatory cytokine is a signalling molecule that induces inflammation in cells, which creates reactive oxygen species. Reactive oxygen species cause oxidative stress in cells, and as a result, the link between lipofuscin and oxidative stress will be investigated.
Lipofuscin formation in melanoma cells and oxidative stress
Cancer and neurodegenerative diseases have a common cause during their formation: oxidative stress (Behrens et al., 2009). However, the interference of specific proteins can dictate whether the problem becomes the uncontrolled death or replication of cells.
ROS are free radicals that are missing an electron in their outermost shell that give them their property of being highly reactive (Davalli et al., 2016). Hence, they can easily disrupt cellular function and induce oxidative stress. Oxidative stress is the state of not being able to balance the level of antioxidants and free radicals in which cellular processes such as the production of proteins can be disrupted. Due to disruptions, proteins partially unfold, which eventually causes the aggregation of proteins inside the cell. In turn, this causes deleterious effects on the proteolytic systems of the cell, which causes lysosomal impairment. During the impairment of lysosomes, protein aggregates inside the lysosomes fluoresce due to lipofuscin formation. Finally, protein aggregation leads to macromolecules being damaged, leading to autophagy in neurodegenerative diseases (de Melo et al., 2013), but the opposite occurs in melanoma when the aggregation occurs (Everts, 2011). In melanoma, when a cell is posed with high ROS levels, p53 is automatically mediated (Davalli et al., 2016). The p53 gene is a tumour suppressor gene that inhibits tumour formation and is usually found to be mutated in cancer cells (National Center for Biotechnology Information (US), 1998). In healthy cells, p53 proteins repair DNA, induce arrest among growth phases and induce apoptosis. However, the p53 protein is mutated in melanoma cells in which autophagy inhibition occurs, thus inducing uncontrolled cell division and lipofuscin being evident in the cancer cells (National Center for Biotechnology Information (US), 1998). Figure 2 displays the oxidative stress pathway for neurodegenerative diseases, and Figure 3 displays the added p53 mechanism evident in the potential oxidative stress pathway for melanoma.
Figure 2. Flow chart demonstrating the mechanisms that are activated which cause the formation of lipofuscin in neurodegenerative diseases.
Note: As observed, the life of the cell ends in autophagy due to the damage caused to macromolecules. This pathway is induced by oxidative stress.
Figure 3. Flow chart demonstrating a modified pathway from the neurodegeneration pathway displayed in Figure 2 to include a p53 mechanism.
Note: As observed, the added p53 mechanism induces the inhibition of autophagy in which the point of lipofuscin accumulation in melanoma cells can be hypothesized.
Medical device innovation: Efficacy and safety
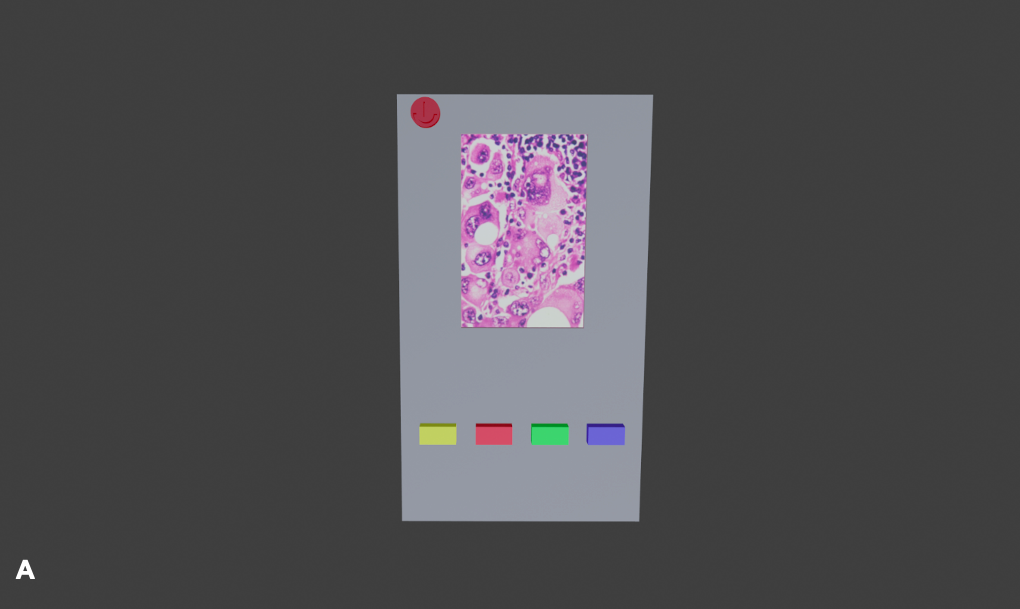
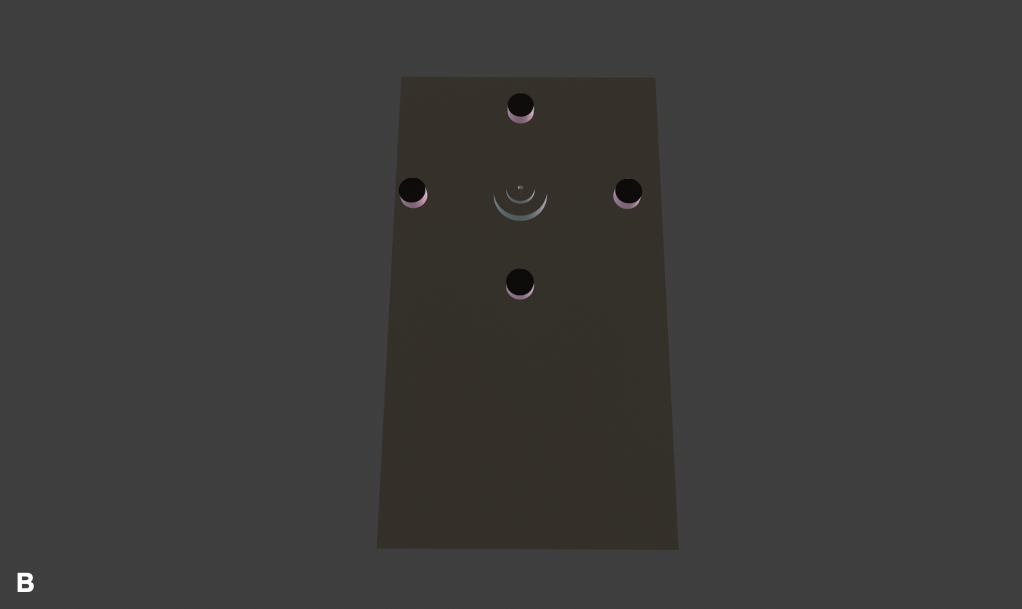
The innovation works through emitting specified wavelengths of near-infrared light onto the lesion. The fluorophores inside the lipofuscin pigment will emit back radiation that the device would be able to detect. The radiation detected would be captured as an image by which the physician will be able to identify lipofuscin. Diagrams of the final device prototype design are represented in Figure 4. Prior to the identification of malignant lesions by measuring lipofuscin levels using a programmed algorithm, the levels of lipofuscin in cancerous cells would be compared to those in non-cancerous cells as a reference. This device is a class 1 device (Center for Devices and Radiological Health, 2020)as it cannot pose any risk to patients since it uses solely near-infrared light for detection, hence being an extremely safe and efficient device.
Note: This device would have the ability to capture images (yellow button), focus on the lesion (dark blue button), save the image and share it with other devices (cyan button) and distinguish whether the lesion is malignant or benign (green button). This device would be constructed using an Arduino microcontroller and other peripheral components (i.e. LCD and push buttons) enclosed in a 3D-printed case.
DISCUSSION
As determined in the Results section, there is relevant evidence proving that it is viable for lipofuscin to be present in melanoma cells. Therefore, it would be useful to investigate further whether lipofuscin tests positive in melanoma cells. Learning if this is true can advance methods of treatment and especially diagnosis. The typical method of diagnosis consists of a visual examination of the mole or tumour by a dermatologist and a biopsy for a confirmed diagnosis. However, the melanoma may have metastasized by the time the biopsy is completed. If lipofuscin is verified as a biomarker for melanoma, a more efficient method of diagnosis would be via autofluorescence imaging through the excitation of molecules within these cells. Excitation wavelengths corresponding to lipofuscin can be used to discover whether lipofuscin exists in melanoma cells. This would be a more versatile diagnostic technique, given that it is minimally invasive. Through this modality of diagnosis, the frequency of misdiagnosed cases of melanoma can be significantly lowered and melanoma can be diagnosed at the earliest stages. In addition, the number of biopsies completed can also be decreased as this additional stage of diagnosis has the potential to narrow down whether a tumour is malignant or not. From the treatment standpoint, lipofuscin can be used as a target for many therapies and can help revolutionize the way we visualize melanoma pathogenesis. Furthermore, researching lipofuscin can improve the understanding of its role in cancer tumorigenesis. Figure 5 illustrates the potential uses of this project in the field of melanoma diagnostics and other disease treatments.
Figure 5. Flowchart illustrating the future pathways for this project and its applications in melanoma diagnostics and treatment along with treatment of other diseases.
The next steps would be to carry out the assays mentioned in the Methods section and to analyze the concentration of lipofuscin within melanoma cells by utilizing fluorescence-activated cell sorting. Essentially, this method of identification would look at the colour, refractive and size factors of lipofuscin in cancer cells. If these assays have successful results, the final step would be to design a device to diagnose melanoma and map the proliferation of melanoma cells. Hence, utilizing lipofuscin as a biomarker would make diagnosis and mapping easier due to its autofluorescent properties.
ACKNOWLEDGEMENTS
I would like to acknowledge Dr. Robert Amelard for mentoring me in the development of the idea for this project, and Dr. Catherine Burns for helping me with my mentor search.
REFERENCES
Behrens, M. I., Lendon, C., & Roe, C. M. (2009). A common biological mechanism in cancer and Alzheimers disease? Current Alzheimer Research, 6(3), 196–204. https://doi.org/10.2174/156720509788486608
Canadian Cancer Society. (n.d.). What is melanoma skin cancer? Cancer. https://www.cancer.ca/en/cancer-information/cancer-type/skin-melanoma/melanoma/?region=on
Center for Devices and Radiological Health. (2020, July 2). Classify Your Medical Device. U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device
Centers for Disease Control and Prevention – Division of Cancer Prevention and Control. (2020, April 9). What Are the Symptoms of Skin Cancer? https://www.cdc.gov/cancer/skin/basic_info/symptoms.htm.
DaCosta, R. S., Kulbatski, I., Lindvere-Teene, L., Starr, D., Blackmore, K., Silver, J. I., Opoku, J., Wu, Y. C., Medeiros, P. J., Xu, W., Xu, L., Wilson, B. C., Rosen, C., & Linden, R. (2015). Point-of-c autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: First-in-human results. PLoS ONE, 10(3). e0116623. https://doi.org/10.1371/journal.pone.0116623
Davalli, P., Mitic, T., Caporali, A., Lauriola, A., & D’Arca, D. (2016). ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Medicine and Cellular Longevity, 2016, 3565127. https://doi.org/10.1155/2016/3565127
de Melo, F. H. M., Molognoni, F., & Jasiulionis, M. G. (2013). The role of oxidative stress in melanoma development, progression and treatment. In L. Davids (Ed.), Recent Advances in the Biology, Therapy and Management of Melanoma (pp. 83-110). InTech. https://doi.org/10.5772/54937
Di Guardo, G. (2015). Lipofuscin, lipofuscin-like pigments and autofluorescence. European Journal of Histochemistry, 59(1), 2485. https://doi.org/10.4081/ejh.2015.2485
Everts, S. (2011, March 28). Cancer: A protein aggregation disease. Chemical and Engineering News. https://cen.acs.org/articles/89/i13/Cancer-Protein-Aggregation-Disease.html
Georgakopoulou, E., Tsimaratou, K., Evangelou, K., Fernandez, M., Zoumpourlis, V., Trougakos, I., Kletsas, D., Bartek, J., Serrano, M., & Gorgoulis, V. (2013). Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging, 5(1), 37-50. https://doi.org/10.18632/aging.100527
Goswami, S., Sharma, S., & Haldar, C. (2013). The oxidative damages caused by ultraviolet radiation type C (UVC) to a tropical rodent Funambulus pennanti: Role of melatonin. Journal of Photochemistry and Photobiology B: Biology, 125, 19–25. https://doi.org/10.1016/j.jphotobiol.2013.04.008
Jegou Penouil, M. H., Gourhant, J. Y., Segretin, C., Weedon, D., & Rosendahl, C. (2014). Non-choroidal yellow melanoma showing positive staining with Sudan Black consistent with the presence of lipofuscin: A case report. Dermatology Practical & Conceptual, 4(2), 45-49. https://doi.org/10.5826/dpc.0402a09
Mayo Clinic. (2020, March 10). Melanoma. https://www.mayoclinic.org/diseases-conditions/melanoma/symptoms-causes/syc-20374884
Mercado, K. (2015, September 15). A clinical guide to fundus autofluorescence. Review of Optometry. https://www.reviewofoptometry.com/article/a-clinical-guide-to-fundus-autofluorescence
National Center for Biotechnology Information (US). (1998, January 1). The p53 tumor suppressor protein. https://www.ncbi.nlm.nih.gov/books/NBK22268/
Santacruz-Perez, C., Tonolli, P. N., Ravagnani, F. G., & Baptista, M. S. (2018). Photochemistry of lipofuscin and the interplay of UVA and visible light in skin photosensitivity. In S. Saha & S. Mondal (Eds.), Photochemistry and Photophysics - Fundamentals to Applications (pp. 89-104). InTech. https://doi.org/10.5772/intechopen.76641
Wu, Y. C., Kulbatski, I., Medeiros, P. J., Maeda, A., Bu, J., Xu, L., Chen, Y., & DaCosta, R. S. (2014). Autofluorescence imaging device for real-time detection and tracking of pathogenic bacteria in a mouse skin wound model: Preclinical feasibility studies. Journal of Biomedical Optics,19(8), 085002. https://doi.org/10.1117/1.JBO.19.8.085002
ABOUT THE AUTHOR
Haarini Suntharalingam
Haarini is a grade 10 student attending the gifted program at Markham District High School. She is interested in studying mechanisms in cancer biology in which she enjoys investigating cancer diagnostics and cancer treatment. She has been participating in regional science fairs since seventh grade in the field of health sciences. She is very passionate about STEM and continues to challenge herself by investigating different forms of cancer for her science fair projects to develop solutions that bridge in the field of health sciences to engineering. In terms of hobbies, she enjoys reading, playing badminton and singing.